Page 51 - Chalcogenide Glasses for Infrared Optics
P. 51
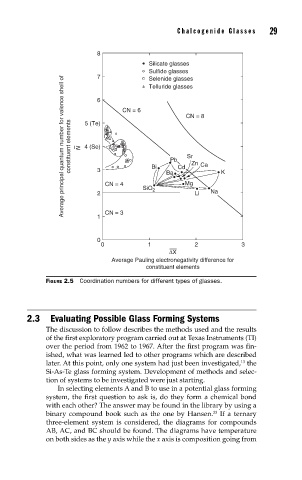
Chalcogenide Glasses 29
8
Silicate glasses
Sulfide glasses
7 Selenide glasses
Telluride glasses
6
CN = 6
CN = 8
constituent elements Average principal quantum number for valence shell of N 4 (Se) Pb Sr Zn Ca
5 (Te)
3
CN = 4 Bi Ba Cd Mg K
SiO 2 Na
2 Li
CN = 3
1
0
0 1 2 3
∆X
Average Pauling electronegativity difference for
constituent elements
FIGURE 2.5 Coordination numbers for different types of glasses.
2.3 Evaluating Possible Glass Forming Systems
The discussion to follow describes the methods used and the results
of the first exploratory program carried out at Texas Instruments (TI)
over the period from 1962 to 1967. After the first program was fin-
ished, what was learned led to other programs which are described
later. At this point, only one system had just been investigated, the
13
Si-As-Te glass forming system. Development of methods and selec-
tion of systems to be investigated were just starting.
In selecting elements A and B to use in a potential glass forming
system, the first question to ask is, do they form a chemical bond
with each other? The answer may be found in the library by using a
33
binary compound book such as the one by Hansen. If a ternary
three-element system is considered, the diagrams for compounds
AB, AC, and BC should be found. The diagrams have temperature
on both sides as the y axis while the x axis is composition going from