Page 34 - Challenges in Corrosion Costs Causes Consequences and Control(2015)
P. 34
12 INTRODUCTION AND FORMS OF CORROSION
8
Corresponds to air saturation
Corrosion rate (gmd) 4
6
2
0
0 2 4 6 10 15 20 25
Concentration of dissolved oxygen, (mL/L)
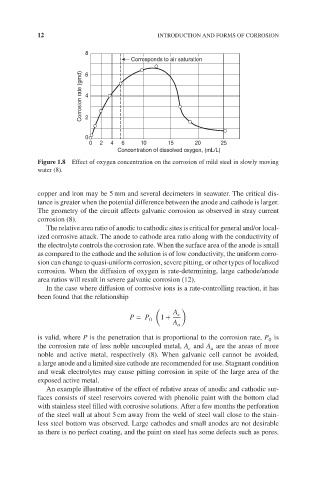
Figure 1.8 Effect of oxygen concentration on the corrosion of mild steel in slowly moving
water (8).
copper and iron may be 5 mm and several decimeters in seawater. The critical dis-
tance is greater when the potential difference between the anode and cathode is larger.
The geometry of the circuit affects galvanic corrosion as observed in stray current
corrosion (8).
The relative area ratio of anodic to cathodic sites is critical for general and/or local-
ized corrosive attack. The anode to cathode area ratio along with the conductivity of
the electrolyte controls the corrosion rate. When the surface area of the anode is small
as compared to the cathode and the solution is of low conductivity, the uniform corro-
sion can change to quasi-uniform corrosion, severe pitting, or other types of localized
corrosion. When the diffusion of oxygen is rate-determining, large cathode/anode
area ratios will result in severe galvanic corrosion (12).
In the case where diffusion of corrosive ions is a rate-controlling reaction, it has
been found that the relationship
( )
A c
P = P 1 +
0
A
a
is valid, where P is the penetration that is proportional to the corrosion rate, P is
0
the corrosion rate of less noble uncoupled metal, A and A are the areas of more
c a
noble and active metal, respectively (8). When galvanic cell cannot be avoided,
a large anode and a limited size cathode are recommended for use. Stagnant condition
and weak electrolytes may cause pitting corrosion in spite of the large area of the
exposed active metal.
An example illustrative of the effect of relative areas of anodic and cathodic sur-
faces consists of steel reservoirs covered with phenolic paint with the bottom clad
with stainless steel filled with corrosive solutions. After a few months the perforation
of the steel wall at about 5 cm away from the weld of steel wall close to the stain-
less steel bottom was observed. Large cathodes and small anodes are not desirable
as there is no perfect coating, and the paint on steel has some defects such as pores.