Page 38 - Challenges in Corrosion Costs Causes Consequences and Control(2015)
P. 38
16 INTRODUCTION AND FORMS OF CORROSION
open and polished hemispherical pits on free surfaces to etched crack-like shapes
within crevices, depending mostly on the rate-controlling reactions during the growth
stage (16). It is also possible that stress corrosion cracking (SCC) and fatigue corro-
sion may result from pitting.
The mechanism of pitting is self-initiating and self-propagating. Pit initiation can
result from a discontinuity in the film, an impurity, different phase, or a scratch on
the surface. The active metal immersed in aerated sodium chloride solution dissolves
in the pit, and the oxygen moves toward the pit. The positively charged iron cations
attract the chloride anions in the pit. The resulting iron chloride hydrolyses and the
sequence of the reactions in the pit are as follows:
+ −
M + Cl → MCl
MCl + H O → MOH + HCl
2
+
HCl → H + Cl −
The hydrolysis of iron chloride regenerates HCl, which can further react with the
iron metal leading to further iron metal dissolution. The pH changes during pitting
corrosion arise from (i) reduction of dissolved oxygen and (ii) reduction of hydrogen.
The precipitation of metal hydroxide at the mouth or the sides of the pit can strengthen
the autocatalytic nature of the penetration of the pit. The rust tubercles on cast iron
indicated pitting in progress, and the inner portion of the bubble was lower in pH and
higher in chloride than outside the bubble.
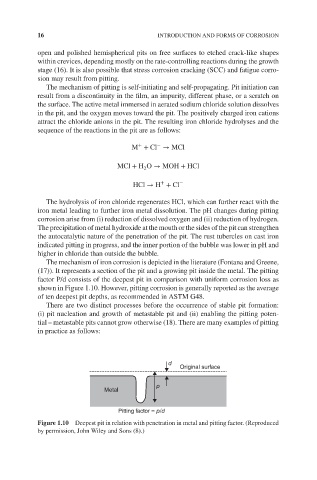
The mechanism of iron corrosion is depicted in the literature (Fontana and Greene,
(17)). It represents a section of the pit and a growing pit inside the metal. The pitting
factor P/d consists of the deepest pit in comparison with uniform corrosion loss as
shown in Figure 1.10. However, pitting corrosion is generally reported as the average
of ten deepest pit depths, as recommended in ASTM G48.
There are two distinct processes before the occurrence of stable pit formation:
(i) pit nucleation and growth of metastable pit and (ii) enabling the pitting poten-
tial – metastable pits cannot grow otherwise (18). There are many examples of pitting
in practice as follows:
d
Original surface
p
Metal
Pitting factor = p/d
Figure 1.10 Deepest pit in relation with penetration in metal and pitting factor. (Reproduced
by permission, John Wiley and Sons (8).)