Page 197 - Chemical and process design handbook
P. 197
Speight_Part II_C 11/7/01 3:08 PM Page 2.138
CAPROLACTAM
o
o
Caprolactam (melting point: 69.3 C, density: 1.02, flash point 125 C,
o
fire point: 140 C), so named because it is derived from the original name
for the C carboxylic acid, caproic acid, is the cyclic amide (lactam) of
6
6-aminocaproic acid.
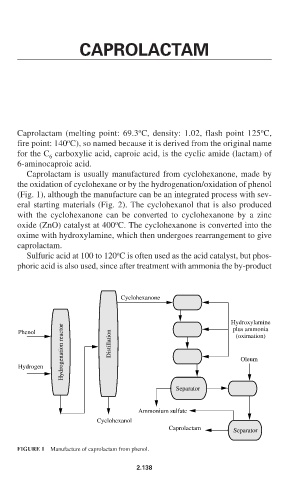
Caprolactam is usually manufactured from cyclohexanone, made by
the oxidation of cyclohexane or by the hydrogenation/oxidation of phenol
(Fig. 1), although the manufacture can be an integrated process with sev-
eral starting materials (Fig. 2). The cyclohexanol that is also produced
with the cyclohexanone can be converted to cyclohexanone by a zinc
o
oxide (ZnO) catalyst at 400 C. The cyclohexanone is converted into the
oxime with hydroxylamine, which then undergoes rearrangement to give
caprolactam.
o
Sulfuric acid at 100 to 120 C is often used as the acid catalyst, but phos-
phoric acid is also used, since after treatment with ammonia the by-product
Cyclohexanone
Hydroxylamine
Hydrogenation reactor Distillation
Phenol plus ammonia
(oximation)
Hydrogen Oleum
Separator
Ammonium sulfate
Cyclohexanol
Caprolactam
Separator
FIGURE 1 Manufacture of caprolactam from phenol.
2.138