Page 24 - Chemical and process design handbook
P. 24
Speight_Part 1_A 11/7/01 3:04 PM Page 1.10
1.10 REACTION TYPES
An equilibrium mixture of the three ethanolamines is produced when eth-
o
ylene oxide is bubbled through 28% aqueous ammonia at 30 to 40 C. By
recirculating the products of the reaction, altering the temperatures, pressures,
and the ratio of ammonia to ethylene oxide, but always having an excess of
ammonia, it is possible to make the desired amine predominate. Diluent gas
also alters the product ratio.
CH CH O +NH → HOCH CH NH + H O
2 2 3 2 2 2 2
monoethanolamine
2CH CH O + NH → (HOCH CH ) NH + 2H O
2 2 3 2 2 2 2
diethanolamine
3CH CH O + NH → (HOCH CH ) N + 3H O
2 3
2
2
2
2
3
triethanolamine
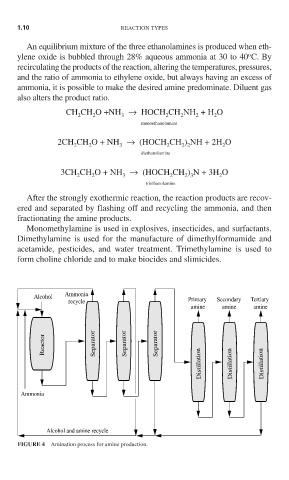
After the strongly exothermic reaction, the reaction products are recov-
ered and separated by flashing off and recycling the ammonia, and then
fractionating the amine products.
Monomethylamine is used in explosives, insecticides, and surfactants.
Dimethylamine is used for the manufacture of dimethylformamide and
acetamide, pesticides, and water treatment. Trimethylamine is used to
form choline chloride and to make biocides and slimicides.
Ammonia
Alcohol Primary Secondary Tertiary
recycle
amine amine amine
Reactor Separator Separator Separator
Distillation Distillation Distillation
Ammonia
Alcohol and amine recycle
FIGURE 4 Amination process for amine production.