Page 44 - Chemical and process design handbook
P. 44
Speight_Part 1_H 11/7/01 3:03 PM Page 1.30
1.30 REACTION TYPES
Many hydrogenation processes are of a proprietary nature, with numerous
combinations of catalysts, temperature, and pressure possible.
Lower pressures and higher temperatures favor dehydrogenation, but
the catalysts used are the same as for hydrogenation.
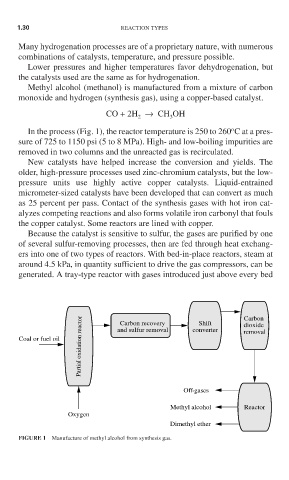
Methyl alcohol (methanol) is manufactured from a mixture of carbon
monoxide and hydrogen (synthesis gas), using a copper-based catalyst.
CO + 2H → CH OH
2 3
o
In the process (Fig. 1), the reactor temperature is 250 to 260 C at a pres-
sure of 725 to 1150 psi (5 to 8 MPa). High- and low-boiling impurities are
removed in two columns and the unreacted gas is recirculated.
New catalysts have helped increase the conversion and yields. The
older, high-pressure processes used zinc-chromium catalysts, but the low-
pressure units use highly active copper catalysts. Liquid-entrained
micrometer-sized catalysts have been developed that can convert as much
as 25 percent per pass. Contact of the synthesis gases with hot iron cat-
alyzes competing reactions and also forms volatile iron carbonyl that fouls
the copper catalyst. Some reactors are lined with copper.
Because the catalyst is sensitive to sulfur, the gases are purified by one
of several sulfur-removing processes, then are fed through heat exchang-
ers into one of two types of reactors. With bed-in-place reactors, steam at
around 4.5 kPa, in quantity sufficient to drive the gas compressors, can be
generated. A tray-type reactor with gases introduced just above every bed
Partial oxidation reactor
Carbon
Shift
Carbon recovery
removal
Coal or fuel oil and sulfur removal converter dioxide
Off-gases
Methyl alcohol Reactor
Oxygen
Dimethyl ether
FIGURE 1 Manufacture of methyl alcohol from synthesis gas.