Page 51 - Chemical and process design handbook
P. 51
Speight_Part 1_N&O 11/7/01 3:02 PM Page 1.37
OXIDATION 1.37
Several oxidative routes are available to change cyclohexane to cyclo-
hexanone, cyclohexanol, and ultimately to adipic acid or caprolactam. If
phenol is hydrogenated, cyclohexanone can be obtained directly; this will
react with hydroxylamine to give cyclohexanone oxime that converts to
caprolactam on acid rearrangement. Cyclohexane can also be converted to
adipic acid, then adiponitrile, which can be converted to hexamethylenedi-
amine. Adipic acid and hexamethylenediamine are used to form nylon 6,6.
This route to hexamethylenediamine is competitive with alternative routes
through butene.
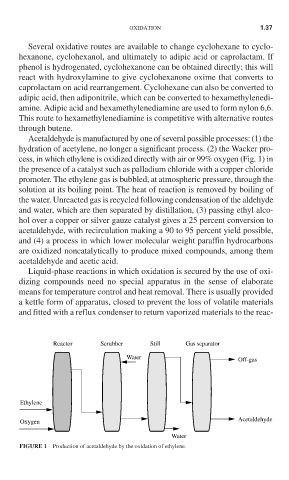
Acetaldehyde is manufactured by one of several possible processes: (1) the
hydration of acetylene, no longer a significant process. (2) the Wacker pro-
cess, in which ethylene is oxidized directly with air or 99% oxygen (Fig. 1) in
the presence of a catalyst such as palladium chloride with a copper chloride
promoter. The ethylene gas is bubbled, at atmospheric pressure, through the
solution at its boiling point. The heat of reaction is removed by boiling of
the water. Unreacted gas is recycled following condensation of the aldehyde
and water, which are then separated by distillation, (3) passing ethyl alco-
hol over a copper or silver gauze catalyst gives a 25 percent conversion to
acetaldehyde, with recirculation making a 90 to 95 percent yield possible,
and (4) a process in which lower molecular weight paraffin hydrocarbons
are oxidized noncatalytically to produce mixed compounds, among them
acetaldehyde and acetic acid.
Liquid-phase reactions in which oxidation is secured by the use of oxi-
dizing compounds need no special apparatus in the sense of elaborate
means for temperature control and heat removal. There is usually provided
a kettle form of apparatus, closed to prevent the loss of volatile materials
and fitted with a reflux condenser to return vaporized materials to the reac-
Reactor Scrubber Still Gas separator
Water
Off-gas
Ethylene
Acetaldehyde
Oxygen
Water
FIGURE 1 Production of acetaldehyde by the oxidation of ethylene.