Page 194 - Chemical equilibria Volume 4
P. 194
170 Chemical Equilibria
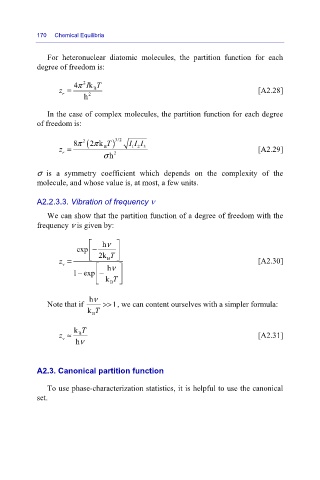
For heteronuclear diatomic molecules, the partition function for each
degree of freedom is:
4π 2 k IT
z = B [A2.28]
r
h 2
In the case of complex molecules, the partition function for each degree
of freedom is:
8π 2 (2 k Tπ ) 3/2 I I I
z = B 1 2 3 [A2.29]
r
σ h 2
σ is a symmetry coefficient which depends on the complexity of the
molecule, and whose value is, at most, a few units.
A2.2.3.3. Vibration of frequency ν
We can show that the partition function of a degree of freedom with the
frequency ν is given by:
⎡ hν ⎤
exp − ⎥
⎢
B ⎦
z = ⎣ 2k T [A2.30]
v
⎡
1exp − hν ⎤ ⎥
−
⎢
B ⎦
⎣ k T
hν
Note that if >> 1, we can content ourselves with a simpler formula:
k T
B
k T
z ≈ B [A2.31]
v
hν
A2.3. Canonical partition function
To use phase-characterization statistics, it is helpful to use the canonical
set.