Page 88 - Chemical equilibria Volume 4
P. 88
64 Chemical Equilibria
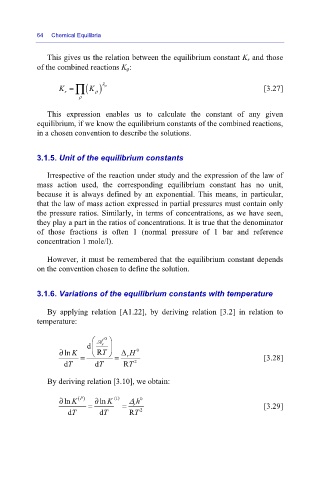
This gives us the relation between the equilibrium constant K r and those
of the combined reactions K ρ:
r ∏
K = ( ) ρ λ [3.27]
K
ρ
ρ
This expression enables us to calculate the constant of any given
equilibrium, if we know the equilibrium constants of the combined reactions,
in a chosen convention to describe the solutions.
3.1.5. Unit of the equilibrium constants
Irrespective of the reaction under study and the expression of the law of
mass action used, the corresponding equilibrium constant has no unit,
because it is always defined by an exponential. This means, in particular,
that the law of mass action expressed in partial pressures must contain only
the pressure ratios. Similarly, in terms of concentrations, as we have seen,
they play a part in the ratios of concentrations. It is true that the denominator
of those fractions is often 1 (normal pressure of 1 bar and reference
concentration 1 mole/l).
However, it must be remembered that the equilibrium constant depends
on the convention chosen to define the solution.
3.1.6. Variations of the equilibrium constants with temperature
By applying relation [A1.22], by deriving relation [3.2] in relation to
temperature:
⎛ 0 ⎞ A
d ⎜ r ⎟
∂ ln K = ⎝ RT ⎠ = Δ H 0 [3.28]
r
dT dT RT 2
By deriving relation [3.10], we obtain:
∂ ln K () = ∂ ln K (I) = Δ r h 0 [3.29]
P
dT dT RT 2