Page 404 - Chemical process engineering design and economics
P. 404
384 Chapter 7
which is sufficient. Therefore, the solution is to use both a coil and a jacket to re-
move the enthalpy of reaction.
The final step is to calculate the mixer power requirement. From Equation
7.4.16, the application that matches this design is reaction with heat transfer. From
Table 7.7, the required power varies from 1.5 to 5 hp/1000 gal. The average power
is 3.25 hp/1000 gal (640 W/m3). Then, according to Equation 7.4.15 the mixer
power,
P = (3.25 hp/1000 gal) (8000 gal) = 26 hp (19.4 kW)
From Table 5.10, a standard-size electric motor is 30 hp (22.4 kW), which
results in a safety factor of 15.4%.
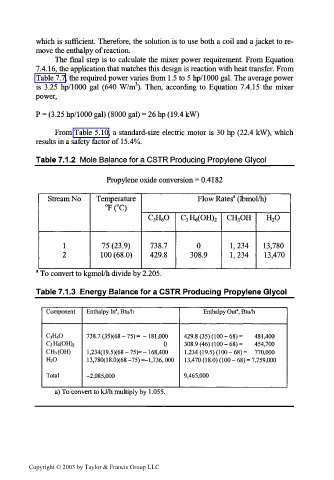
Table 7.1.2 Mole Balance for a CSTR Producing Propylene Glycol____
Propylene oxide conversion = 0.4182
a
Stream No Temperature Flow Rates (Ibmol/h)
°F(°C)
C 3H 60 C 3H 6(OH) 2 CH 3OH' H 2O
1 75 (23.9) 738.7 0 1,234 13,780
2 100 (68.0) 429.8 308.9 1,234 13,470
a To convert to kgmol/h divide by 2.205.
Table 7.1.3 Energy Balance for a CSTR Producing Propylene Glycol
Component Enthalpy In", Btu/h Enthalpy Out', Btu/h
C 3H60 738.7 (35)(68- 75)= -181,000 429.8 (35) (100 -68)= 481,400
C 3H 6(OH) 2 0 308.9 (46) (1 00 - 68) = 454,700
CH 3(OH) 1,234(19.5)(68 - 75)=- 168,400 1,234 (19.5) (100 -68)= 770,000
H 20 1 3,780(1 8.0)(68 -75) =-1,736, 000 13,470 (18.0) (100 - 68) = 7,759,000
Total -2,085,000 9,465,000
a) To convert to kJ/h multiply by 1.055.
Copyright © 2003 by Taylor & Francis Group LLC