Page 164 - Chiral Separation Techniques
P. 164
142 5 Membranes in Chiral Separations
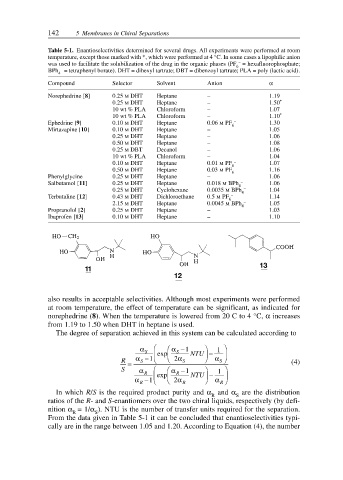
Table 5-1. Enantioselectivities determined for several drugs. All experiments were performed at room
temperature, except those marked with *, which were performed at 4 °C. In some cases a lipophilic anion
was used to facilitate the solubilization of the drug in the organic phases (PF 6 – = hexafluorophosphate;
–
BPh = tetraphenyl borate). DHT = dihexyl tartrate; DBT = dibenzoyl tartrate; PLA = poly (lactic acid).
4
Compound Selector Solvent Anion α
Norephedrine [8] 0.25 M DHT Heptane – 1.19
0.25 M DHT Heptane – 1.50 *
10 wt % PLA Chloroform – 1.07
10 wt % PLA Chloroform – 1.10 *
Ephedrine [9] 0.10 M DHT Heptane 0.06 M PF – 1.30
6
Mirtazapine [10] 0.10 M DHT Heptane – 1.05
0.25 M DHT Heptane – 1.06
0.50 M DHT Heptane – 1.08
0.25 M DBT Decanol – 1.06
10 wt % PLA Chloroform – 1.04
0.10 M DHT Heptane 0.01 M PF – 1.07
6
0.50 M DHT Heptane 0.03 M PF – 1.16
6
Phenylglycine 0.25 M DHT Heptane – 1.06
Salbutamol [11] 0.25 M DHT Heptane 0.018 M BPh – 1.06
4
0.25 M DHT Cyclohexane 0.0035 M BPh 4 – 1.04
Terbutaline [12] 0.43 M DHT Dichloroethane 0.5 M PF – 1.14
6
2.15 M DHT Heptane 0.0045 M BPh – 1.05
4
Propranolol [2] 0.25 M DHT Heptane – 1.03
Ibuprofen [13] 0.10 M DHT Heptane – 1.10
also results in acceptable selectivities. Although most experiments were performed
at room temperature, the effect of temperature can be significant, as indicated for
norephedrine (8). When the temperature is lowered from 20 C to 4 °C, α increases
from 1.19 to 1.50 when DHT in heptane is used.
The degree of separation achieved in this system can be calculated according to
α S α S −1 NTU − 1
α − exp 2 α α S
1
R = S S (4)
S α R α R −1 NTU − 1
α − exp 2 α α
1
R R R
In which R/S is the required product purity and α and α are the distribution
R S
ratios of the R- and S-enantiomers over the two chiral liquids, respectively (by defi-
nition α = 1/α ). NTU is the number of transfer units required for the separation.
R S
From the data given in Table 5-1 it can be concluded that enantioselectivities typi-
cally are in the range between 1.05 and 1.20. According to Equation (4), the number