Page 163 - Chiral Separation Techniques
P. 163
5.3 Membrane-Assisted Chiral Separations 141
5.3.2 Liquid–Membrane Fractionation
As described above, the application of classical liquid– liquid extractions often
results in extreme flow ratios. To avoid this, a completely symmetrical system has
been developed at Akzo Nobel in the early 1990s [64, 65]. In this system, a sup-
ported liquid–membrane separates two miscible chiral liquids containing opposite
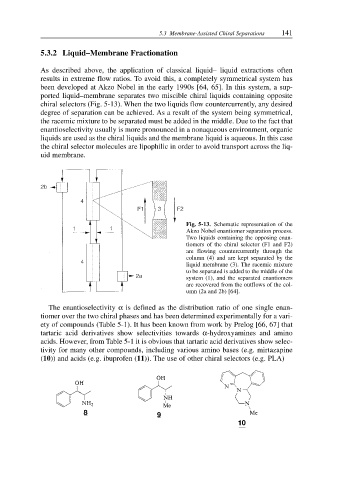
chiral selectors (Fig. 5-13). When the two liquids flow countercurrently, any desired
degree of separation can be achieved. As a result of the system being symmetrical,
the racemic mixture to be separated must be added in the middle. Due to the fact that
enantioselectivity usually is more pronounced in a nonaqueous environment, organic
liquids are used as the chiral liquids and the membrane liquid is aqueous. In this case
the chiral selector molecules are lipophilic in order to avoid transport across the liq-
uid membrane.
Fig. 5-13. Schematic representation of the
Akzo Nobel enantiomer separation process.
Two liquids containing the opposing enan-
tiomers of the chiral selector (F1 and F2)
are flowing countercurrently through the
column (4) and are kept separated by the
liquid membrane (3). The racemic mixture
to be separated is added to the middle of the
system (1), and the separated enantiomers
are recovered from the outflows of the col-
umn (2a and 2b) [64].
The enantioselectivity α is defined as the distribution ratio of one single enan-
tiomer over the two chiral phases and has been determined experimentally for a vari-
ety of compounds (Table 5-1). It has been known from work by Prelog [66, 67] that
tartaric acid derivatives show selectivities towards α-hydroxyamines and amino
acids. However, from Table 5-1 it is obvious that tartaric acid derivatives show selec-
tivity for many other compounds, including various amino bases (e.g. mirtazapine
(10)) and acids (e.g. ibuprofen (11)). The use of other chiral selectors (e.g. PLA)