Page 167 - Chiral Separation Techniques
P. 167
5.3 Membrane-Assisted Chiral Separations 145
5.3.3 Micellar-Enhanced Ultrafiltration
Ultrafiltration of micellar solutions combines the high permeate flows commonly
found in ultrafiltration systems with the possibility of removing molecules indepen-
dent of their size, since micelles can specifically solubilize or bind low molecular
weight components. Characteristics of this separation technique, known as micellar-
enhanced ultrafiltration (MEUF), are that micelles bind specific compounds and sub-
sequent ultrafiltration separates the surrounding aqueous phase from the micelles
[70]. The pore size of the UF membrane must be chosen such, that the micelles are
retained but the unbound components can pass the membrane freely. Alternatively,
proteins such as BSA have been used in stead of micelles to obtain similar enan-
tioselective aggregates [71].
For the separation of amino acids, the applicability of this principle has been
explored. For the separation of racemic phenylalanine, an amphiphilic amino acid
derivative, l-5-cholesteryl glutamate (14) has been used as a chiral co-surfactant in
micelles of the nonionic surfactant Serdox NNP TM 10. Copper(II) ions are added for
the formation of ternary complexes between phenylalanine and the amino acid co-
surfactant. The basis for the separation is the difference in stability between the
ternary complexes formed with d- or l-phenylalanine, respectively. The basic princi-
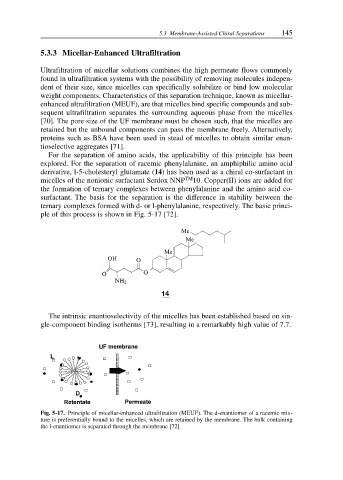
ple of this process is shown in Fig. 5-17 [72].
The intrinsic enantioselectivity of the micelles has been established based on sin-
gle-component binding isotherms [73], resulting in a remarkably high value of 7.7.
Fig. 5-17. Principle of micellar-enhanced ultrafiltration (MEUF). The d-enantiomer of a racemic mix-
ture is preferentially bound to the micelles, which are retained by the membrane. The bulk containing
the l-enantiomer is separated through the membrane [72].