Page 301 - Chiral Separation Techniques
P. 301
280 10 The Use of SMB for the Manufacture of Enantiopure Drug Substances: From …
100
99
98
Raffinate without
97
Purity (%) 96 Extract without
adjustment
95
adjustment
94
adjustment
93 Raffinate with
92 Extract with
adjustment
91
90
2.9 3 3.1 3.15 3.3 3.4
Retention factor of the more retained compound
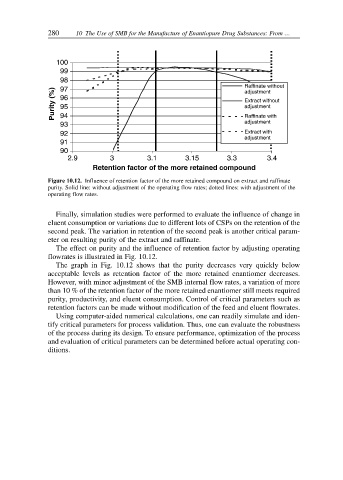
Figure 10.12. Influence of retention factor of the more retained compound on extract and raffinate
purity. Solid line: without adjustment of the operating flow rates; dotted lines: with adjustment of the
operating flow rates.
Finally, simulation studies were performed to evaluate the influence of change in
eluent consumption or variations due to different lots of CSPs on the retention of the
second peak. The variation in retention of the second peak is another critical param-
eter on resulting purity of the extract and raffinate.
The effect on purity and the influence of retention factor by adjusting operating
flowrates is illustrated in Fig. 10.12.
The graph in Fig. 10.12 shows that the purity decreases very quickly below
acceptable levels as retention factor of the more retained enantiomer decreases.
However, with minor adjustment of the SMB internal flow rates, a variation of more
than 10 % of the retention factor of the more retained enantiomer still meets required
purity, productivity, and eluent consumption. Control of critical parameters such as
retention factors can be made without modification of the feed and eluent flowrates.
Using computer-aided numerical calculations, one can readily simulate and iden-
tify critical parameters for process validation. Thus, one can evaluate the robustness
of the process during its design. To ensure performance, optimization of the process
and evaluation of critical parameters can be determined before actual operating con-
ditions.