Page 134 - Color Atlas of Biochemistry
P. 134
Energy Metabolism 125
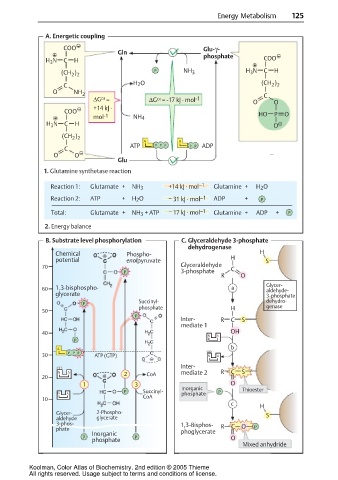
A. Energetic coupling
COO Glu-γ-
Gln
H 3 N C H phosphate COO
(CH ) P NH 3 H 3 N C H
22
C H 2 O (CH )
22
O NH 2 C
.
OI
∆G = ∆G = -17 kJ mol -1 O O
OI
+14 kJ .
COO
mol -1 NH 4 HO P O
H 3 N C H O
(CH )
22
A A
ATP PP P P P ADP
C
O O –
Glu
1. Glutamine synthetase reaction
.
Reaction 1: Glutamate + NH 3 +14 kJ mol –1 Glutamine + H 2 O
.
Reaction 2: ATP + H 2 O – 31 kJ mol –1 ADP + P
.
Total: Glutamate + NH 3 + ATP – 17 kJ mol –1 Glutamine + ADP + P
2. Energy balance
B. Substrate level phosphorylation C. Glyceraldehyde 3-phosphate
dehydrogenase
Chemical Phospho- H
potential enolpyruvate H S
70 Glyceraldehyde C
P 3-phosphate
R O
Glycer-
60 1,3-bisphospho- a aldehyde-
glycerate 3-phosphate
Succinyl- dehydro-
P
phosphate H genase
50
P
Inter- R C S
mediate 1
40 OH
N A
P
b
A
30 PP P ATP (GTP) N A
Inter-
N A 2 CoA mediate 2 R C S
20
1 3 O
Inorganic Thioester
P Succinyl- P
N A phosphate
10 CoA
c
H
Glycer- 2-Phospho- S
aldehyde glycerate
3-phos- 1,3-Bisphos- R C O
phate phoglycerate P
Inorganic
P P O
phosphate
Mixed anhydride
Koolman, Color Atlas of Biochemistry, 2nd edition © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.