Page 26 - Color Atlas of Biochemistry
P. 26
Physical Chemistry 17
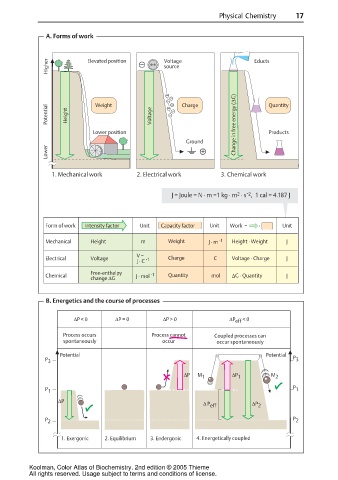
A. Forms of work
Higher Elevated position Voltage Educts
source
Potential Height Weight Voltage Charge Quantity
Lower position Change in free energy (∆G) Products
Ground
Lower
1. Mechanical work 2. Electrical work 3. Chemical work
2
-2
J = Joule = N · m =1 kg · m · s , 1 cal = 4.187 J
Form of work Intensity factor Unit Capacity factor Unit Work = · Unit
Mechanical Height m Weight J · m -1 Height · Weight J
V =
Electrical Voltage -1 Charge C Voltage · Charge J
J · C
Free-enthalpy
Chemical J · mol -1 Quantity mol ∆G · Quantity J
change ∆G
B. Energetics and the course of processes
∆P < 0 ∆P = 0 ∆P > 0 ∆P eff < 0
Process occurs Process cannot Coupled processes can
spontaneously occur occur spontaneously
Potential Potential
P 3 P 3
∆P M 1 ∆P 1 M 2
P 1 P 1
∆P
∆ P eff ∆P 2
P 2 P 2
1. Exergonic 2. Equilibrium 3. Endergonic 4. Energetically coupled
Koolman, Color Atlas of Biochemistry, 2nd edition © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.