Page 28 - Color Atlas of Biochemistry
P. 28
Physical Chemistry 19
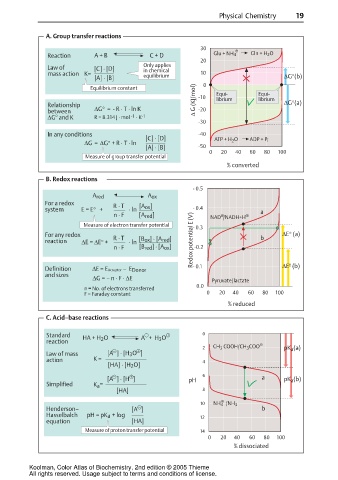
A. Group transfer reactions
30
Reaction A + B C + D Glu + NH 4 Gln + H 2 O
20
Only applies
Law of [C] · [D]
mass action K= in chemical 10
[A] · [B] equilibrium ∆G°(b)
Equilibrium constant 0 Equi- Equi-
Relationship ∆ G (KJ/mol) -10 librium librium ∆G°(a)
between ∆G° = - R · T · ln K -20
0
∆G and K R = 8.314 J · mol · K -1
-1
-30
In any conditions -40
[C] · [D] ATP + H O ADP + P i
∆G= ∆G° + R · T · ln 2
[A] · [B] -50
0 20 40 60 80 100
Measure of group transfer potential
% converted
B. Redox reactions
- 0.5
A red A ox
For a redox R · T [A ox ]
system E = E° + · ln - 0.4 a
n · F [A red ] NAD /NADH+H
Measure of electron transfer potential - 0.3
For any redox ∆E° (a)
reaction ∆E = ∆E° + R · T · ln [B ox ] · [A red ] Redox potential E (V) b
n · F [B red ] · [A ox ] -0.2
Definition ∆E = EAcceptor – E Donor - 0.1 ∆Eº (b)
and sizes
∆G = – n · F · ∆E
Pyruvate/lactate
0.0
n = No. of electrons transferred
F = Faraday constant 0 20 40 60 80 100
% reduced
C. Acid–base reactions
Standard HA + H 2 O A + H 3 O 0
reaction
2 CH COOH/CH COO pK (a)
3
3
a
Law of mass [A ] · [H 3 O ]
action K = 4
[HA] · [H 2 O]
6
[A ] · [H ] pH a pK (b)
a
Simplified K =
a
[HA] 8
10 NH 4 /NH 3
Henderson– [A ] b
Hasselbalch pH = pK a + log 12
equation [HA]
Measure of proton transfer potential 14
0 20 40 60 80 100
% dissociated
Koolman, Color Atlas of Biochemistry, 2nd edition © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.