Page 32 - Color Atlas of Biochemistry
P. 32
Physical Chemistry 23
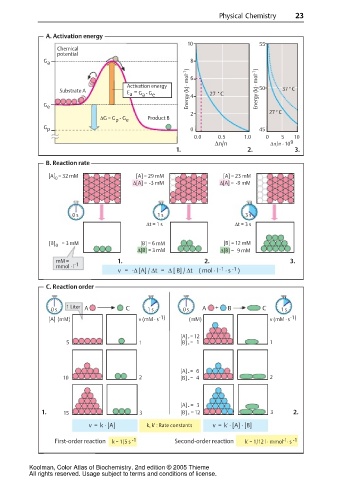
A. Activation energy
10 55
Chemical
potential
G a 8
Energy (kJ · mol -1 ) Energy (kJ · mol -1 ) 50 37 C
Activation energy 6
˚
Substrate A E = G - G e 4 27 C
a
˚
a
G e
˚
2 27 C
∆G = G - G e Product B
p
G p 0 45
0.0 0.5 1.0 0 5 10
∆n/n ∆n/n · 10 9
1. 2. 3.
B. Reaction rate
[A] 0 = 32 mM [A] = 29 mM [A] = 23 mM
∆[A] = -3 mM ∆[A] = -9 mM
0 s 1 s 3 s
∆t = 1 s ∆t = 3 s
[B] = 3 mM [B] = 6 mM [B] = 12 mM
0
∆[B] = 3 mM ∆[B] = 9 mM
mM = 1. 2. 3.
mmol · l -1
-1
-1
v = -∆ [A] / ∆t = ∆ [ B] / ∆t ( mol · l · s )
C. Reaction order
1 Liter A C A + B C
0 s 1 s 0 s 1 s
[A] (mM) v (mM · s -1) (mM) v (mM · s -1)
[A] = 12
5 1 [B] ˚ = 1 1
˚
[A] = 6
10 2 [B] ˚ = 4 2
˚
[A] = 3
1. 15 3 [B] ˚ = 12 3 2.
˚
v = k · [A] k, k' : Rate constants v = k' · [A] · [B]
-1
First-order reaction k = 1/5 s -1 Second-order reaction k' = 1/12 l · mmol · s -1
Koolman, Color Atlas of Biochemistry, 2nd edition © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.