Page 34 - Color Atlas of Biochemistry
P. 34
Physical Chemistry 25
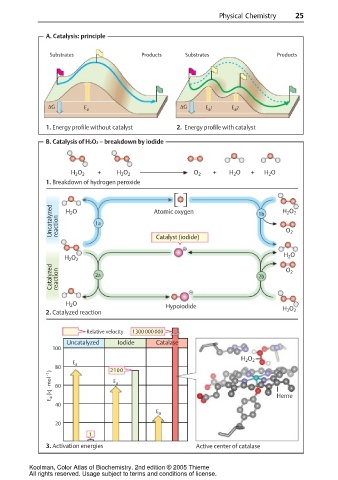
A. Catalysis: principle
Substrates Products Substrates Products
∆G E a ∆G E 1 E 2
a
a
1. Energy profile without catalyst 2. Energy profile with catalyst
B. Catalysis of H2O2 – breakdown by iodide
H O 2 + H O 2 O 2 + H O + H O
2
2
2
2
1. Breakdown of hydrogen peroxide
Uncatalyzed reaction H O 1a Atomic oxygen 1b H O 2
2
2
O
2
Catalyst (iodide)
H O
H O 2 2
2
Catalyzed reaction 2a 2b O 2
H O Hypoiodide
2
2
2. Catalyzed reaction H O 2
Relative velocity 1300000000
Uncatalyzed Iodide Catalase
100
H O 2
2
E a
80 2100
E a (kJ · mol -1 ) 60 E a Heme
40
E a
20
1
3. Activation energies Active center of catalase
Koolman, Color Atlas of Biochemistry, 2nd edition © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.