Page 42 - Color Atlas of Biochemistry
P. 42
Physical Chemistry 33
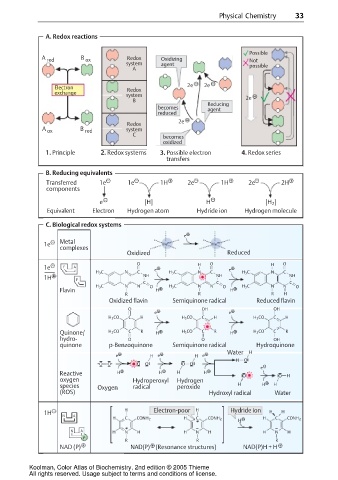
A. Redox reactions
Possible
A red B ox Redox Oxidizing Not
system agent possible
A
Electron Redox 2e 2e
exchange
system 2e
B
Reducing
becomes agent
reduced
2e
Redox
A ox B red system
C
becomes
oxidized
1. Principle 2. Redox systems 3. Possible electron 4. Redox series
3. transfers
B. Reducing equivalents
Transferred 1e 1e 1H 2e 1H 2e 2H
components
e [H] H [H 2 ]
Equivalent Electron Hydrogen atom Hydride ion Hydrogen molecule
C. Biological redox systems
e
Metal n+ m+
1e Me Me
complexes
Oxidized Reduced
O H O H O
1e F A
H 3 C N C e H 3 C N C e H 3 C N C
1H F NH NH NH
C C C
H 3 C N N O H 3 C N N O H 3 C N N O
Flavin H H
R R R H
Oxidized flavin Semiquinone radical Reduced flavin
O OH OH
e e
H 3 CO C H H 3 CO C H H 3 CO C H
Quinone/ H 3 CO C R H H 3 CO C R H H 3 CO C R
hydro- O O OH
quinone p-Benzoquinone Semiquinone radical Hydroquinone
Water H
e H e H e
H O
O O O O O O
e
Reactive H H H H O O H
oxygen Hydroperoxyl Hydrogen
species Oxygen radical peroxide H H H
(ROS) Hydroxyl radical Water
H Electron-poor H Hydride ion
1H H H
N A
H C CONH 2 H C CONH 2 H C CONH 2
H
N A H N H H N H H N H
P
R R R
NAD (P) NAD(P) (Resonance structures) NAD(P)H + H
Koolman, Color Atlas of Biochemistry, 2nd edition © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.