Page 40 - Color Atlas of Biochemistry
P. 40
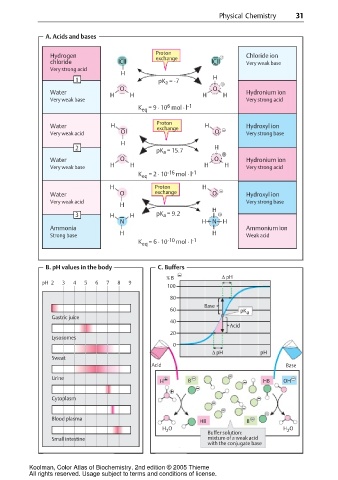
Physical Chemistry 31
A. Acids and bases
Proton
Hydrogen exchange Chloride ion
chloride Cl Cl Very weak base
Very strong acid
H
1 pK = -7 H
a
O O
Water H H H H Hydronium ion
Very weak base Very strong acid
6
K eq = 9 · 10 mol · l -1
Water H Proton H Hydroxyl ion
exchange
Very weak acid O O Very strong base
H
2 pK = 15.7 H
a
Water O O Hydronium ion
Very weak base H H H H Very strong acid
K eq = 2 · 10 -16 mol · l -1
H Proton H
Water O exchange O Hydroxyl ion
Very weak acid Very strong base
H
H
3 H H pK = 9.2
a
N H N H
Ammonia Ammonium ion
Strong base H H Weak acid
K eq = 6 · 10 -10 mol · l -1
B. pH values in the body C. Buffers
% B ∆ pH
pH 2 3 4 5 6 7 8 9
100
80
Base
60 pK a
Gastric juice
40
Acid
20
Lysosomes
0
∆ pH pH
Sweat
Acid Base
Urine
H B HB OH
Cytoplasm
Blood plasma
HB B
H O H O
2
2
Buffer solution:
Small intestine mixture of a weak acid
with the conjugate base
Koolman, Color Atlas of Biochemistry, 2nd edition © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.