Page 185 - Corrosion Engineering Principles and Practice
P. 185
160 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 161
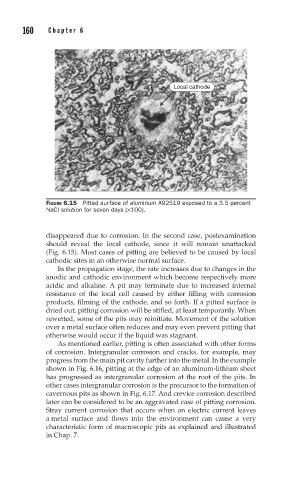
Local cathode
FIGURE 6.15 Pitted surface of aluminum A92519 exposed to a 3.5 percent
NaCl solution for seven days (×100).
disappeared due to corrosion. In the second case, postexamination
should reveal the local cathode, since it will remain unattacked
(Fig. 6.15). Most cases of pitting are believed to be caused by local
cathodic sites in an otherwise normal surface.
In the propagation stage, the rate increases due to changes in the
anodic and cathodic environment which become respectively more
acidic and alkaline. A pit may terminate due to increased internal
resistance of the local cell caused by either filling with corrosion
products, filming of the cathode, and so forth. If a pitted surface is
dried out, pitting corrosion will be stifled, at least temporarily. When
rewetted, some of the pits may reinitiate. Movement of the solution
over a metal surface often reduces and may even prevent pitting that
otherwise would occur if the liquid was stagnant.
As mentioned earlier, pitting is often associated with other forms
of corrosion. Intergranular corrosion and cracks, for example, may
progress from the main pit cavity further into the metal. In the example
shown in Fig. 6.16, pitting at the edge of an aluminum-lithium sheet
has progressed as intergranular corrosion at the root of the pits. In
other cases intergranular corrosion is the precursor to the formation of
cavernous pits as shown in Fig. 6.17. And crevice corrosion described
later can be considered to be an aggravated case of pitting corrosion.
Stray current corrosion that occurs when an electric current leaves
a metal surface and flows into the environment can cause a very
characteristic form of macroscopic pits as explained and illustrated
in Chap. 7.