Page 217 - Corrosion Engineering Principles and Practice
P. 217
192 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 193
1.2
1
Molybdenum
Relative mass loss 0.6 Copper
0.8
0.4
Chromium
0.2
0
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1
Material content (%)
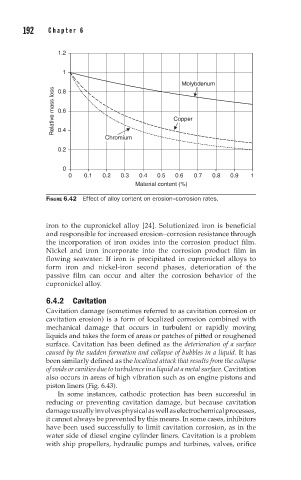
FIGURE 6.42 Effect of alloy content on erosion–corrosion rates.
iron to the cupronickel alloy [24]. Solutionized iron is beneficial
and responsible for increased erosion–corrosion resistance through
the incorporation of iron oxides into the corrosion product film.
Nickel and iron incorporate into the corrosion product film in
flowing seawater. If iron is precipitated in cupronickel alloys to
form iron and nickel-iron second phases, deterioration of the
passive film can occur and alter the corrosion behavior of the
cupronickel alloy.
6.4.2 Cavitation
Cavitation damage (sometimes referred to as cavitation corrosion or
cavitation erosion) is a form of localized corrosion combined with
mechanical damage that occurs in turbulent or rapidly moving
liquids and takes the form of areas or patches of pitted or roughened
surface. Cavitation has been defined as the deterioration of a surface
caused by the sudden formation and collapse of bubbles in a liquid. It has
been similarly defined as the localized attack that results from the collapse
of voids or cavities due to turbulence in a liquid at a metal surface. Cavitation
also occurs in areas of high vibration such as on engine pistons and
piston liners (Fig. 6.43).
In some instances, cathodic protection has been successful in
reducing or preventing cavitation damage, but because cavitation
damage usually involves physical as well as electrochemical processes,
it cannot always be prevented by this means. In some cases, inhibitors
have been used successfully to limit cavitation corrosion, as in the
water side of diesel engine cylinder liners. Cavitation is a problem
with ship propellers, hydraulic pumps and turbines, valves, orifice