Page 216 - Corrosion Engineering Principles and Practice
P. 216
190 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 191
1000
Ferritic and
martensitic
stainless steels Austenitic-ferritic
duplex steels
Austenitic Cr-Ni-
Mo steels
Corrosion rate (gm –2 Day –1 ) 100 Austenitic Mn
steels
10
Austenitic
special steels
1
0 20 40 60
–1
Flow velocity (m s )
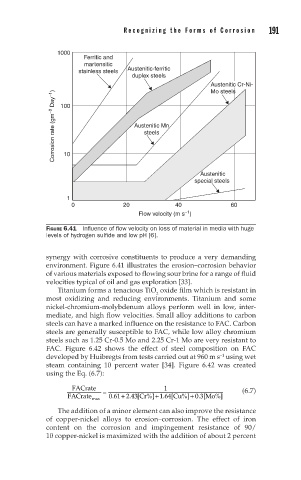
FIGURE 6.41 Influence of flow velocity on loss of material in media with huge
levels of hydrogen sulfide and low pH [6].
synergy with corrosive constituents to produce a very demanding
environment. Figure 6.41 illustrates the erosion–corrosion behavior
of various materials exposed to flowing sour brine for a range of fluid
velocities typical of oil and gas exploration [33].
Titanium forms a tenacious TiO oxide film which is resistant in
2
most oxidizing and reducing environments. Titanium and some
nickel-chromium-molybdenum alloys perform well in low, inter-
mediate, and high flow velocities. Small alloy additions to carbon
steels can have a marked influence on the resistance to FAC. Carbon
steels are generally susceptible to FAC, while low alloy chromium
steels such as 1.25 Cr-0.5 Mo and 2.25 Cr-1 Mo are very resistant to
FAC. Figure 6.42 shows the effect of steel composition on FAC
developed by Huibregts from tests carried out at 960 m s using wet
−1
steam containing 10 percent water [34]. Figure 6.42 was created
using the Eq. (6.7):
FACrate = 1 (6.7)
FACrate max 0 61. + 2 43[ Cr% + 1.64[Cu%]+ 0.3[Mo%]
+
.
]
The addition of a minor element can also improve the resistance
of copper-nickel alloys to erosion–corrosion. The effect of iron
content on the corrosion and impingement resistance of 90/
10 copper-nickel is maximized with the addition of about 2 percent