Page 236 - Corrosion Engineering Principles and Practice
P. 236
210 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 211
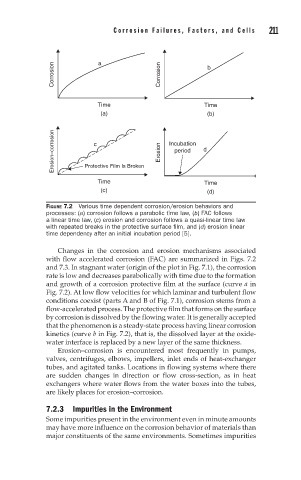
Corrosion a Corrosion b
Time Time
(a) (b)
Erosion–corrosion Protective Film Is Broken Erosion Incubation d
c
period
Time Time
(c) (d)
FIGURE 7.2 Various time dependent corrosion/erosion behaviors and
processes: (a) corrosion follows a parabolic time law, (b) FAC follows
a linear time law, (c) erosion and corrosion follows a quasi-linear time law
with repeated breaks in the protective surface film, and (d) erosion linear
time dependency after an initial incubation period [5].
Changes in the corrosion and erosion mechanisms associated
with flow accelerated corrosion (FAC) are summarized in Figs. 7.2
and 7.3. In stagnant water (origin of the plot in Fig. 7.1), the corrosion
rate is low and decreases parabolically with time due to the formation
and growth of a corrosion protective film at the surface (curve a in
Fig. 7.2). At low flow velocities for which laminar and turbulent flow
conditions coexist (parts A and B of Fig. 7.1), corrosion stems from a
flow-accelerated process. The protective film that forms on the surface
by corrosion is dissolved by the flowing water. It is generally accepted
that the phenomenon is a steady-state process having linear corrosion
kinetics (curve b in Fig. 7.2), that is, the dissolved layer at the oxide-
water interface is replaced by a new layer of the same thickness.
Erosion–corrosion is encountered most frequently in pumps,
valves, centrifuges, elbows, impellers, inlet ends of heat-exchanger
tubes, and agitated tanks. Locations in flowing systems where there
are sudden changes in direction or flow cross-section, as in heat
exchangers where water flows from the water boxes into the tubes,
are likely places for erosion–corrosion.
7.2.3 Impurities in the Environment
Some impurities present in the environment even in minute amounts
may have more influence on the corrosion behavior of materials than
major constituents of the same environments. Sometimes impurities