Page 235 - Corrosion Engineering Principles and Practice
P. 235
210 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 211
short life of galvanized steel water heater tanks. Electrochemical
measurements showed that in many cases, iron was anodic to zinc
above 75°C, whereas zinc was anodic to iron at temperatures below
60°C. This explained why zinc offered no cathodic protection above
75°C, and why red water and premature perforation of galvanized
water tanks occurred so readily at higher temperatures. This particular
problem was partly solved by using magnesium sacrificial anodes or
protective coatings, and by the replacement with new alloys.
7.2.2 Fluid Velocity Effects
Unless otherwise protected, metals generally owe their corrosion
resistance to a tightly adherent, protective film that builds up on the
metal surface by corrosion processes. This film may consist of reaction
products, adsorbed gases, or a combination of these. Any mechanical
disturbance of this protective film can stimulate attack of the
underlying metals until either the protective film is reestablished, or
the metal has been corroded away. The mechanical disturbance itself
can be caused by abrasion, impingement, turbulence, or cavitation.
Carbon steel pipe carrying water, for example, is usually protected
by a film of rust that slows down the rate of mass transfer of dissolved
oxygen to the pipe wall. The resulting corrosion rates are typically less
than 1 mm/y. The removal of the film by flowing sand slurry has been
shown to raise corrosion rates tenfold to approximately 10 mm/y [4].
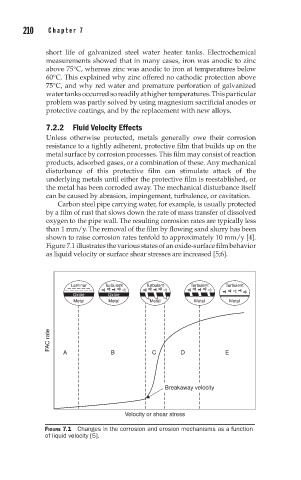
Figure 7.1 illustrates the various states of an oxide-surface film behavior
as liquid velocity or surface shear stresses are increased [5;6].
Laminar Turbulent Turbulent Turbulent Turbulent
Oxide Oxide
Metal Metal Metal Metal Metal
FAC rate A B C D E
Breakaway velocity
Velocity or shear stress
FIGURE 7.1 Changes in the corrosion and erosion mechanisms as a function
of liquid velocity [5].