Page 269 - Corrosion Engineering Principles and Practice
P. 269
242 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 243
Hydrated
alumina
7
Relative volume 5
3
1
Al Al O 3 Al O ·H O Al(OH) 3
2
2
3
2
Oxide type
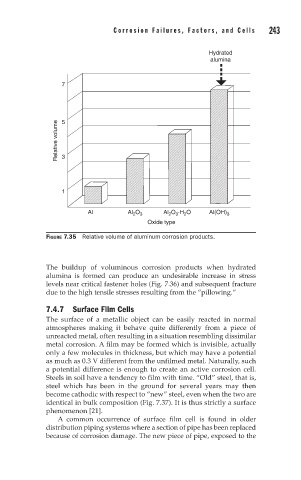
FIGURE 7.35 Relative volume of aluminum corrosion products.
The buildup of voluminous corrosion products when hydrated
alumina is formed can produce an undesirable increase in stress
levels near critical fastener holes (Fig. 7.36) and subsequent fracture
due to the high tensile stresses resulting from the “pillowing.”
7.4.7 Surface Film Cells
The surface of a metallic object can be easily reacted in normal
atmospheres making it behave quite differently from a piece of
unreacted metal, often resulting in a situation resembling dissimilar
metal corrosion. A film may be formed which is invisible, actually
only a few molecules in thickness, but which may have a potential
as much as 0.3 V different from the unfilmed metal. Naturally, such
a potential difference is enough to create an active corrosion cell.
Steels in soil have a tendency to film with time. “Old” steel, that is,
steel which has been in the ground for several years may then
become cathodic with respect to “new” steel, even when the two are
identical in bulk composition (Fig. 7.37). It is thus strictly a surface
phenomenon [21].
A common occurrence of surface film cell is found in older
distribution piping systems where a section of pipe has been replaced
because of corrosion damage. The new piece of pipe, exposed to the