Page 266 - Corrosion Engineering Principles and Practice
P. 266
240 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 241
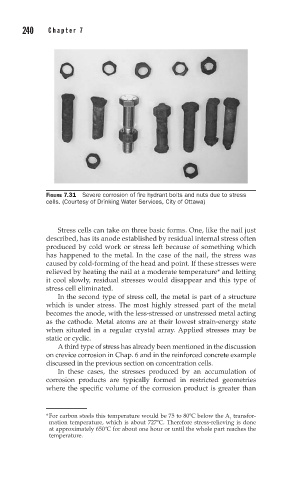
FIGURE 7.31 Severe corrosion of fire hydrant bolts and nuts due to stress
cells. (Courtesy of Drinking Water Services, City of Ottawa)
Stress cells can take on three basic forms. One, like the nail just
described, has its anode established by residual internal stress often
produced by cold work or stress left because of something which
has happened to the metal. In the case of the nail, the stress was
caused by cold-forming of the head and point. If these stresses were
relieved by heating the nail at a moderate temperature* and letting
it cool slowly, residual stresses would disappear and this type of
stress cell eliminated.
In the second type of stress cell, the metal is part of a structure
which is under stress. The most highly stressed part of the metal
becomes the anode, with the less-stressed or unstressed metal acting
as the cathode. Metal atoms are at their lowest strain-energy state
when situated in a regular crystal array. Applied stresses may be
static or cyclic.
A third type of stress has already been mentioned in the discussion
on crevice corrosion in Chap. 6 and in the reinforced concrete example
discussed in the previous section on concentration cells.
In these cases, the stresses produced by an accumulation of
corrosion products are typically formed in restricted geometries
where the specific volume of the corrosion product is greater than
* For carbon steels this temperature would be 75 to 80ºC below the A transfor-
1
mation temperature, which is about 727ºC. Therefore stress-relieving is done
at approximately 650ºC for about one hour or until the whole part reaches the
temperature.