Page 261 - Corrosion Engineering Principles and Practice
P. 261
234 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 235
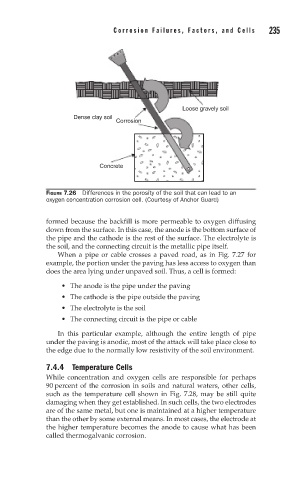
Loose gravely soil
Dense clay soil
Corrosion
Concrete
FIGURE 7.26 Differences in the porosity of the soil that can lead to an
oxygen concentration corrosion cell. (Courtesy of Anchor Guard)
formed because the backfill is more permeable to oxygen diffusing
down from the surface. In this case, the anode is the bottom surface of
the pipe and the cathode is the rest of the surface. The electrolyte is
the soil, and the connecting circuit is the metallic pipe itself.
When a pipe or cable crosses a paved road, as in Fig. 7.27 for
example, the portion under the paving has less access to oxygen than
does the area lying under unpaved soil. Thus, a cell is formed:
• The anode is the pipe under the paving
• The cathode is the pipe outside the paving
• The electrolyte is the soil
• The connecting circuit is the pipe or cable
In this particular example, although the entire length of pipe
under the paving is anodic, most of the attack will take place close to
the edge due to the normally low resistivity of the soil environment.
7.4.4 Temperature Cells
While concentration and oxygen cells are responsible for perhaps
90 percent of the corrosion in soils and natural waters, other cells,
such as the temperature cell shown in Fig. 7.28, may be still quite
damaging when they get established. In such cells, the two electrodes
are of the same metal, but one is maintained at a higher temperature
than the other by some external means. In most cases, the electrode at
the higher temperature becomes the anode to cause what has been
called thermogalvanic corrosion.