Page 260 - Corrosion Engineering Principles and Practice
P. 260
234 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 235
contact with the solution containing the higher concentration of DO
will become cathodic to surfaces in contact with a lower concentration
of DO causing these surfaces to suffer accelerated corrosion as anodes
in an oxygen concentration cell.
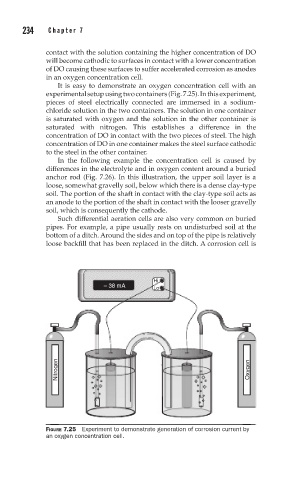
It is easy to demonstrate an oxygen concentration cell with an
experimental setup using two containers (Fig. 7.25). In this experiment,
pieces of steel electrically connected are immersed in a sodium-
chloride solution in the two containers. The solution in one container
is saturated with oxygen and the solution in the other container is
saturated with nitrogen. This establishes a difference in the
concentration of DO in contact with the two pieces of steel. The high
concentration of DO in one container makes the steel surface cathodic
to the steel in the other container.
In the following example the concentration cell is caused by
differences in the electrolyte and in oxygen content around a buried
anchor rod (Fig. 7.26). In this illustration, the upper soil layer is a
loose, somewhat gravelly soil, below which there is a dense clay-type
soil. The portion of the shaft in contact with the clay-type soil acts as
an anode to the portion of the shaft in contact with the looser gravelly
soil, which is consequently the cathode.
Such differential aeration cells are also very common on buried
pipes. For example, a pipe usually rests on undisturbed soil at the
bottom of a ditch. Around the sides and on top of the pipe is relatively
loose backfill that has been replaced in the ditch. A corrosion cell is
Hi
– 38 mA Lo
Nitrogen Oxygen
FIGURE 7.25 Experiment to demonstrate generation of corrosion current by
an oxygen concentration cell.