Page 258 - Corrosion Engineering Principles and Practice
P. 258
232 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 233
In the example shown in Fig. 7.22, the main difference in the
environment surrounding the steel anchor is due to the variation of
pH between the soil typically acidic and the basic pH in the concrete
anchorage (pH > 10). Because this higher pH is protective to steel, it
naturally follows that the steel in concrete becomes the cathode
drawing an anodic current from the adjacent steel in the soil.
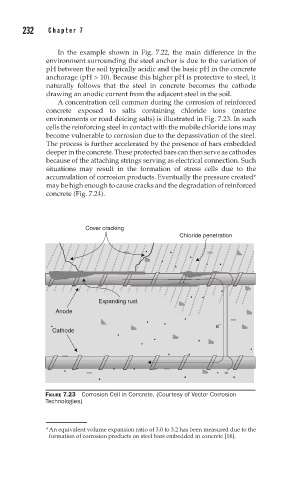
A concentration cell common during the corrosion of reinforced
concrete exposed to salts containing chloride ions (marine
environments or road deicing salts) is illustrated in Fig. 7.23. In such
cells the reinforcing steel in contact with the mobile chloride ions may
become vulnerable to corrosion due to the depassivation of the steel.
The process is further accelerated by the presence of bars embedded
deeper in the concrete. These protected bars can then serve as cathodes
because of the attaching strings serving as electrical connection. Such
situations may result in the formation of stress cells due to the
accumulation of corrosion products. Eventually the pressure created*
may be high enough to cause cracks and the degradation of reinforced
concrete (Fig. 7.24).
Cover cracking
Chloride penetration
Expanding rust
Anode
e –
Cathode
FIGURE 7.23 Corrosion Cell in Concrete. (Courtesy of Vector Corrosion
Technologies)
* An equivalent volume expansion ratio of 3.0 to 3.2 has been measured due to the
formation of corrosion products on steel bars embedded in concrete [16].