Page 257 - Corrosion Engineering Principles and Practice
P. 257
230 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 231
Electrical insulators
Grade
Corrosion
Ground
copper rod
Concrete
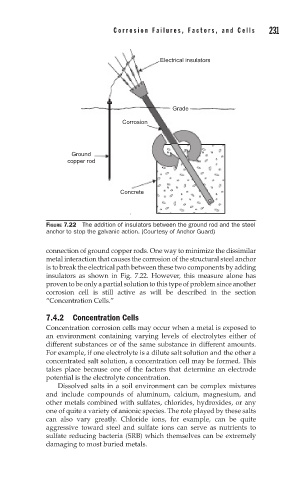
FIGURE 7.22 The addition of insulators between the ground rod and the steel
anchor to stop the galvanic action. (Courtesy of Anchor Guard)
connection of ground copper rods. One way to minimize the dissimilar
metal interaction that causes the corrosion of the structural steel anchor
is to break the electrical path between these two components by adding
insulators as shown in Fig. 7.22. However, this measure alone has
proven to be only a partial solution to this type of problem since another
corrosion cell is still active as will be described in the section
“Concentration Cells.”
7.4.2 Concentration Cells
Concentration corrosion cells may occur when a metal is exposed to
an environment containing varying levels of electrolytes either of
different substances or of the same substance in different amounts.
For example, if one electrolyte is a dilute salt solution and the other a
concentrated salt solution, a concentration cell may be formed. This
takes place because one of the factors that determine an electrode
potential is the electrolyte concentration.
Dissolved salts in a soil environment can be complex mixtures
and include compounds of aluminum, calcium, magnesium, and
other metals combined with sulfates, chlorides, hydroxides, or any
one of quite a variety of anionic species. The role played by these salts
can also vary greatly. Chloride ions, for example, can be quite
aggressive toward steel and sulfate ions can serve as nutrients to
sulfate reducing bacteria (SRB) which themselves can be extremely
damaging to most buried metals.