Page 408 - Corrosion Engineering Principles and Practice
P. 408
376 C h a p t e r 9 A t m o s p h e r i c C o r r o s i o n 377
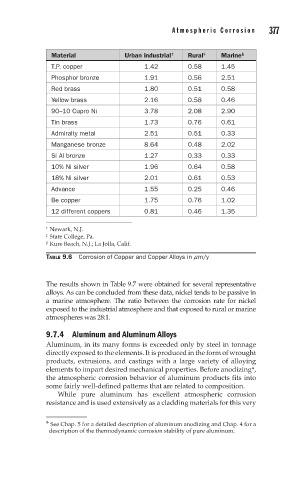
Material Urban industrial † Rural ‡ Marine §
T.P. copper 1.42 0.58 1.45
Phosphor bronze 1.91 0.56 2.51
Red brass 1.80 0.51 0.58
Yellow brass 2.16 0.58 0.46
90–10 Cupro Ni 3.78 2.08 2.90
Tin brass 1.73 0.76 0.61
Admiralty metal 2.51 0.51 0.33
Manganese bronze 8.64 0.48 2.02
Si Al bronze 1.27 0.33 0.33
10% Ni silver 1.96 0.64 0.58
18% Ni silver 2.01 0.61 0.53
Advance 1.55 0.25 0.46
Be copper 1.75 0.76 1.02
12 different coppers 0.81 0.46 1.35
† Newark, N.J.
‡ State College, Pa.
§ Kure Beach, N.J.; La Jolla, Calif.
TABLE 9.6 Corrosion of Copper and Copper Alloys in mm/y
The results shown in Table 9.7 were obtained for several representative
alloys. As can be concluded from these data, nickel tends to be passive in
a marine atmosphere. The ratio between the corrosion rate for nickel
exposed to the industrial atmosphere and that exposed to rural or marine
atmospheres was 28:1.
9.7.4 Aluminum and Aluminum Alloys
Aluminum, in its many forms is exceeded only by steel in tonnage
directly exposed to the elements. It is produced in the form of wrought
products, extrusions, and castings with a large variety of alloying
elements to impart desired mechanical properties. Before anodizing*,
the atmospheric corrosion behavior of aluminum products fits into
some fairly well-defined patterns that are related to composition.
While pure aluminum has excellent atmospheric corrosion
resistance and is used extensively as a cladding materials for this very
* See Chap. 5 for a detailed description of aluminum anodizing and Chap. 4 for a
description of the thermodynamic corrosion stability of pure aluminum.