Page 409 - Corrosion Engineering Principles and Practice
P. 409
378 C h a p t e r 9 A t m o s p h e r i c C o r r o s i o n 379
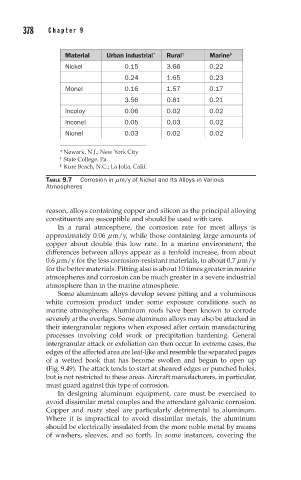
Material Urban industrial * Rural † Marine ‡
Nickel 0.15 3.66 0.22
0.24 1.65 0.23
Monel 0.16 1.57 0.17
3.56 0.81 0.21
Incoloy 0.06 0.02 0.02
Inconel 0.05 0.03 0.02
Nionel 0.03 0.02 0.02
* Newark, N.J.; New York City
† State College, Pa.
‡ Kure Beach, N.C.; La Jolla, Calif.
TABLE 9.7 Corrosion in mm/y of Nickel and Its Alloys in Various
Atmospheres
reason, alloys containing copper and silicon as the principal alloying
constituents are susceptible and should be used with care.
In a rural atmosphere, the corrosion rate for most alloys is
approximately 0.06 m m/y, while those containing large amounts of
copper about double this low rate. In a marine environment, the
differences between alloys appear as a tenfold increase, from about
0.6 m m/y for the less corrosion-resistant materials, to about 0.7 m m/y
for the better materials. Pitting also is about 10 times greater in marine
atmospheres and corrosion can be much greater in a severe industrial
atmosphere than in the marine atmosphere.
Some aluminum alloys develop severe pitting and a voluminous
white corrosion product under some exposure conditions such as
marine atmospheres. Aluminum roofs have been known to corrode
severely at the overlaps. Some aluminum alloys may also be attacked in
their intergranular regions when exposed after certain manufacturing
processes involving cold work or precipitation hardening. General
intergranular attack or exfoliation can then occur. In extreme cases, the
edges of the affected area are leaf-like and resemble the separated pages
of a wetted book that has become swollen and begun to open up
(Fig. 9.49). The attack tends to start at sheared edges or punched holes,
but is not restricted to these areas. Aircraft manufacturers, in particular,
must guard against this type of corrosion.
In designing aluminum equipment, care must be exercised to
avoid dissimilar metal couples and the attendant galvanic corrosion.
Copper and rusty steel are particularly detrimental to aluminum.
Where it is impractical to avoid dissimilar metals, the aluminum
should be electrically insulated from the more noble metal by means
of washers, sleeves, and so forth. In some instances, covering the