Page 47 - Corrosion Engineering Principles and Practice
P. 47
28 C h a p t e r 2 C o r r o s i o n B a s i c s 29
(a)
(a)
(b)
(b)
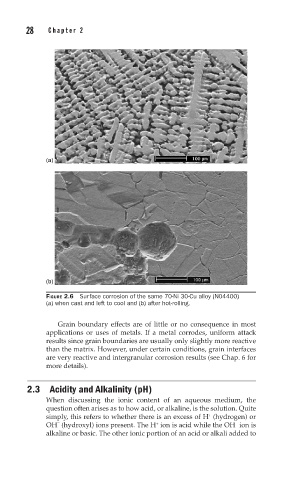
FIGURE 2.6 Surface corrosion of the same 70-Ni 30-Cu alloy (N04400)
(a) when cast and left to cool and (b) after hot-rolling.
Grain boundary effects are of little or no consequence in most
applications or uses of metals. If a metal corrodes, uniform attack
results since grain boundaries are usually only slightly more reactive
than the matrix. However, under certain conditions, grain interfaces
are very reactive and intergranular corrosion results (see Chap. 6 for
more details).
2.3 Acidity and Alkalinity (pH)
When discussing the ionic content of an aqueous medium, the
question often arises as to how acid, or alkaline, is the solution. Quite
+
simply, this refers to whether there is an excess of H (hydrogen) or
−
+
OH (hydroxyl) ions present. The H ion is acid while the OH ion is
_
alkaline or basic. The other ionic portion of an acid or alkali added to