Page 49 - Corrosion Engineering Principles and Practice
P. 49
30 C h a p t e r 2 C o r r o s i o n B a s i c s 31
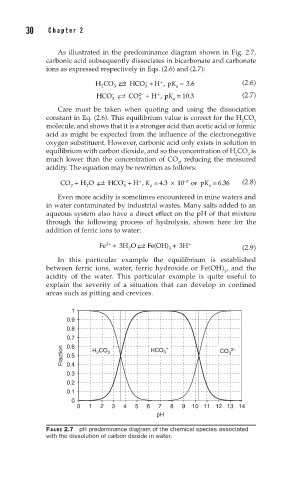
As illustrated in the predominance diagram shown in Fig. 2.7,
carbonic acid subsequently dissociates in bicarbonate and carbonate
ions as expressed respectively in Eqs. (2.6) and (2.7):
−
+
H CO HCO + H , pK a = 3.6 (2.6)
3
2
3
−
+
HCO CO 2− + H , pK a = 10.3 (2.7)
3
3
Care must be taken when quoting and using the dissociation
constant in Eq. (2.6). This equilibrium value is correct for the H CO
3
2
molecule, and shows that it is a stronger acid than acetic acid or formic
acid as might be expected from the influence of the electronegative
oxygen substituent. However, carbonic acid only exists in solution in
equilibrium with carbon dioxide, and so the concentration of H CO is
2
3
much lower than the concentration of CO , reducing the measured
2
acidity. The equation may be rewritten as follows:
−
CO + H O HCO + H , K = 4 3 × 10 7 − or pK = 6..36 (2.8)
.
+
2 2 3 a a
Even more acidity is sometimes encountered in mine waters and
in water contaminated by industrial wastes. Many salts added to an
aqueous system also have a direct effect on the pH of that mixture
through the following process of hydrolysis, shown here for the
addition of ferric ions to water:
Fe 3+ + 3H O Fe(OH) + 3H + (2.9)
2 3
In this particular example the equilibrium is established
between ferric ions, water, ferric hydroxide or Fe(OH) , and the
3
acidity of the water. This particular example is quite useful to
explain the severity of a situation that can develop in confined
areas such as pitting and crevices.
1
0.9
0.8
0.7
0.6
Fraction 0.5 H CO 3 HCO 3 – CO 3 2–
2
0.4
0.3
0.2
0.1
0
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14
pH
FIGURE 2.7 pH predominance diagram of the chemical species associated
with the dissolution of carbon dioxide in water.