Page 55 - Corrosion Engineering Principles and Practice
P. 55
36 C h a p t e r 3 C o r r o s i o n E l e c t r o c h e m i s t r y 37
FIGURE 3.1
Schematic of e –
a Daniell cell.
Current
Copper
Zinc
Zn 2+ Cu 2+
SO 4 2– SO 4 2–
own soluble sulfates, a technical point that is critical in order to keep
the voltage of a Daniell cell relatively constant [1]. The same goal can
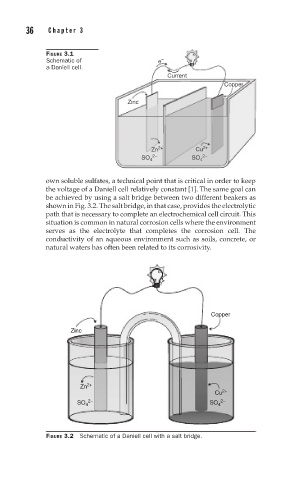
be achieved by using a salt bridge between two different beakers as
shown in Fig. 3.2. The salt bridge, in that case, provides the electrolytic
path that is necessary to complete an electrochemical cell circuit. This
situation is common in natural corrosion cells where the environment
serves as the electrolyte that completes the corrosion cell. The
conductivity of an aqueous environment such as soils, concrete, or
natural waters has often been related to its corrosivity.
Copper
Zinc
Zn 2+
Cu 2+
SO 4 2– SO 4 2–
FIGURE 3.2 Schematic of a Daniell cell with a salt bridge.