Page 56 - Corrosion Engineering Principles and Practice
P. 56
36 C h a p t e r 3 C o r r o s i o n E l e c t r o c h e m i s t r y 37
The short-hand description in Eq. (3.3) is valid for both cells
shown in Figs. 3.1 and 3.2. Such a description is often used to simplify
textual reference to such cells.
−
+
+
−
−
+
( )Zn/Zn , SO 2 4 (Conc ) 1 //Cu , SO 2 4 (Conc 2 ) ) /Cu( ) (3.3)
2
2
Conc and Conc in Eq. (3.3) indicate respectively the concentration
2
1
of zinc sulfate and copper sulfate that may differ in the two half-cells
while the two slanted bars (//) describe the presence of a separator.
The same short-hand description also identifies the zinc electrode as
the anode that is negative in the case of a spontaneous reaction and
the copper cathode as positive.
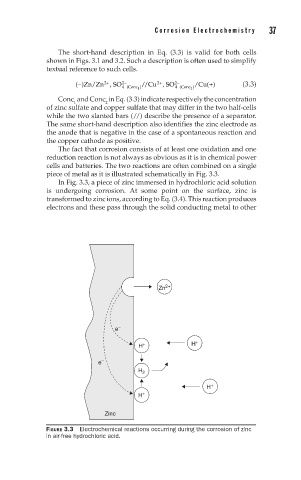
The fact that corrosion consists of at least one oxidation and one
reduction reaction is not always as obvious as it is in chemical power
cells and batteries. The two reactions are often combined on a single
piece of metal as it is illustrated schematically in Fig. 3.3.
In Fig. 3.3, a piece of zinc immersed in hydrochloric acid solution
is undergoing corrosion. At some point on the surface, zinc is
transformed to zinc ions, according to Eq. (3.4). This reaction produces
electrons and these pass through the solid conducting metal to other
Zn 2+
e –
H + H +
e –
H 2
H +
H +
Zinc
FIGURE 3.3 Electrochemical reactions occurring during the corrosion of zinc
in air-free hydrochloric acid.