Page 59 - Corrosion Engineering Principles and Practice
P. 59
40 C h a p t e r 3 C o r r o s i o n E l e c t r o c h e m i s t r y 41
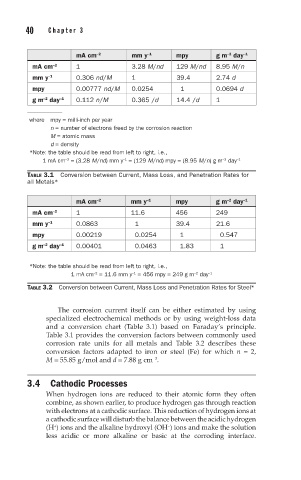
mA cm –2 mm y –1 mpy g m day –1
–2
mA cm –2 1 3.28 M/nd 129 M/nd 8.95 M/n
mm y –1 0.306 nd/M 1 39.4 2.74 d
mpy 0.00777 nd/M 0.0254 1 0.0694 d
g m day –1 0.112 n/M 0.365 /d 14.4 /d 1
–2
where mpy = milli-inch per year
n = number of electrons freed by the corrosion reaction
M = atomic mass
d = density
*Note: the table should be read from left to right, i.e.,
1 mA cm = (3.28 M/nd) mm y = (129 M/nd) mpy = (8.95 M/n) g m day –1
–1
–2
–2
TABLE 3.1 Conversion between Current, Mass Loss, and Penetration Rates for
all Metals*
mA cm –2 mm y –1 mpy g m day –1
–2
mA cm –2 1 11.6 456 249
mm y –1 0.0863 1 39.4 21.6
mpy 0.00219 0.0254 1 0.547
g m day –1 0.00401 0.0463 1.83 1
–2
*Note: the table should be read from left to right, i.e.,
–2
1 mA cm = 11.6 mm y = 456 mpy = 249 g m day –1
–1
–2
TABLE 3.2 Conversion between Current, Mass Loss and Penetration Rates for Steel*
The corrosion current itself can be either estimated by using
specialized electrochemical methods or by using weight-loss data
and a conversion chart (Table 3.1) based on Faraday’s principle.
Table 3.1 provides the conversion factors between commonly used
corrosion rate units for all metals and Table 3.2 describes these
conversion factors adapted to iron or steel (Fe) for which n = 2,
M = 55.85 g/mol and d = 7.88 g cm .
−3
3.4 Cathodic Processes
When hydrogen ions are reduced to their atomic form they often
combine, as shown earlier, to produce hydrogen gas through reaction
with electrons at a cathodic surface. This reduction of hydrogen ions at
a cathodic surface will disturb the balance between the acidic hydrogen
+
(H ) ions and the alkaline hydroxyl (OH ) ions and make the solution
−
less acidic or more alkaline or basic at the corroding interface.