Page 63 - Corrosion Engineering Principles and Practice
P. 63
44 C h a p t e r 3 C o r r o s i o n E l e c t r o c h e m i s t r y 45
Zn 2+
Zn 2+
Zn 2+
e –
H +
H +
H 2
e – H +
H +
e – H +
H +
e – O
H +
e – O 2
H +
e – O
H + H O H
H +
Zinc
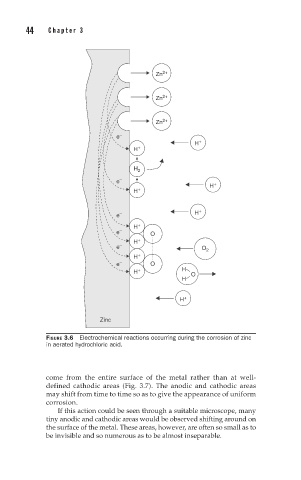
FIGURE 3.6 Electrochemical reactions occurring during the corrosion of zinc
in aerated hydrochloric acid.
come from the entire surface of the metal rather than at well-
defined cathodic areas (Fig. 3.7). The anodic and cathodic areas
may shift from time to time so as to give the appearance of uniform
corrosion.
If this action could be seen through a suitable microscope, many
tiny anodic and cathodic areas would be observed shifting around on
the surface of the metal. These areas, however, are often so small as to
be invisible and so numerous as to be almost inseparable.