Page 632 - Corrosion Engineering Principles and Practice
P. 632
594 C h a p t e r 1 4 P r o t e c t i v e C o a t i n g s 595
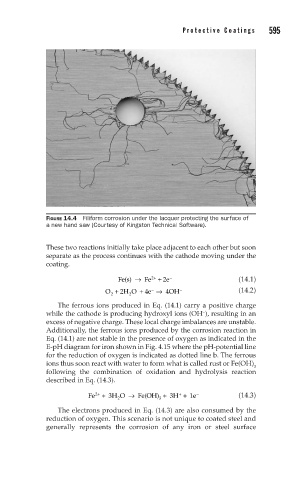
FIGURE 14.4 Filiform corrosion under the lacquer protecting the surface of
a new hand saw (Courtesy of Kingston Technical Software).
These two reactions initially take place adjacent to each other but soon
separate as the process continues with the cathode moving under the
coating.
Fe(s) → Fe 2+ + 2e (14.1)
−
O + 2H O + 4e → 4OH (14.2)
−
−
2
2
The ferrous ions produced in Eq. (14.1) carry a positive charge
−
while the cathode is producing hydroxyl ions (OH ), resulting in an
excess of negative charge. These local charge imbalances are unstable.
Additionally, the ferrous ions produced by the corrosion reaction in
Eq. (14.1) are not stable in the presence of oxygen as indicated in the
E-pH diagram for iron shown in Fig. 4.15 where the pH-potential line
for the reduction of oxygen is indicated as dotted line b. The ferrous
ions thus soon react with water to form what is called rust or Fe(OH)
3
following the combination of oxidation and hydrolysis reaction
described in Eq. (14.3).
Fe 2+ + 3H O → Fe(OH) + 3H + 1e − (14.3)
+
2
3
The electrons produced in Eq. (14.3) are also consumed by the
reduction of oxygen. This scenario is not unique to coated steel and
generally represents the corrosion of any iron or steel surface