Page 69 - Corrosion Engineering Principles and Practice
P. 69
50 C h a p t e r 4 C o r r o s i o n T h e r m o d y n a m i c s 51
+1.11 V Hi
Lo
Copper
Zinc
Zn 2+ Cu 2+
SO 4 2– SO 4 2–
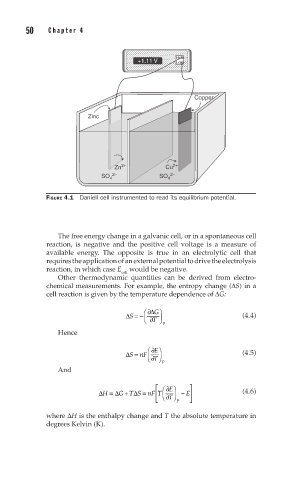
FIGURE 4.1 Daniell cell instrumented to read its equilibrium potential.
The free energy change in a galvanic cell, or in a spontaneous cell
reaction, is negative and the positive cell voltage is a measure of
available energy. The opposite is true in an electrolytic cell that
requires the application of an external potential to drive the electrolysis
reaction, in which case E would be negative.
cell
Other thermodynamic quantities can be derived from electro-
chemical measurements. For example, the entropy change (∆S) in a
cell reaction is given by the temperature dependence of ∆G:
∆S = − ∂ ∂ ∆G (4.4)
T
P
Hence
∆S = nF ∂ E (4.5)
∂
T
P
And
∆H = ∆G T ∆S = nF T ∂ E − E (4.6)
+
∂ T P
where ∆H is the enthalpy change and T the absolute temperature in
degrees Kelvin (K).