Page 72 - Corrosion Engineering Principles and Practice
P. 72
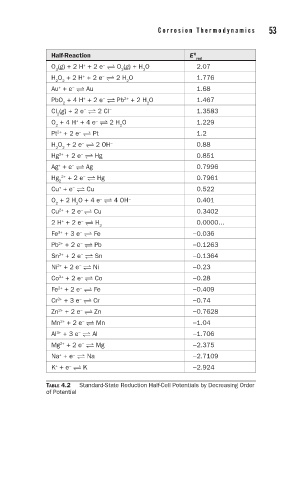
52 C h a p t e r 4 C o r r o s i o n T h e r m o d y n a m i c s 53
Half-Reaction E 0
red
O (g) + 2 H + 2 e O (g) + H O 2.07
+
−
3 2 2
+
H O + 2 H + 2 e 2 H O 1.776
−
2 2 2
−
Au + e Au 1.68
+
PbO + 4 H + 2 e Pb + 2 H O 1.467
−
+
2+
2 2
−
Cl (g) + 2 e 2 Cl − 1.3583
2
O + 4 H + 4 e 2 H O 1.229
+
−
2 2
Pt + 2 e Pt 1.2
2+
−
H O + 2 e 2 OH 0.88
−
−
2 2
Hg + 2 e Hg 0.851
−
2+
Ag + e Ag 0.7996
+
−
Hg 2+ + 2 e Hg 0.7961
−
2
Cu + e Cu 0.522
+
−
−
O + 2 H O + 4 e 4 OH − 0.401
2 2
−
2+
Cu + 2 e Cu 0.3402
2 H + 2 e H 2 0.0000...
+
−
Fe + 3 e Fe −0.036
3+
−
−
Pb + 2 e Pb −0.1263
2+
Sn + 2 e Sn −0.1364
2+
−
Ni + 2 e Ni −0.23
2+
−
−
2+
Co + 2 e Co −0.28
2+
−
Fe + 2 e Fe −0.409
Cr + 3 e Cr −0.74
3+
−
Zn + 2 e Zn −0.7628
2+
−
Mn + 2 e Mn −1.04
2+
−
Al + 3 e Al −1.706
3+
−
Mg + 2 e Mg −2.375
−
2+
+
Na + e Na −2.7109
−
−
+
K + e K −2.924
TABLE 4.2 Standard-State Reduction Half-Cell Potentials by Decreasing Order
of Potential