Page 76 - Corrosion Engineering Principles and Practice
P. 76
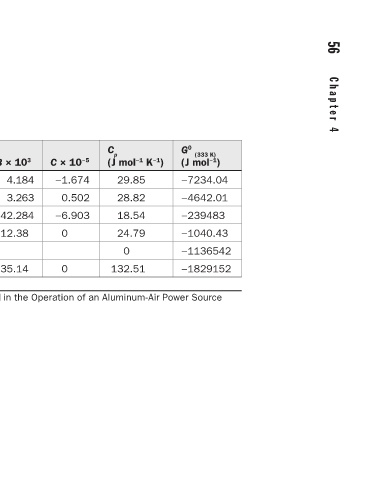
56 C h a p t e r 4 C o r r o s i o n T h e r m o d y n a m i c s 57
G 0 (333 K) ) (J mol −1 −7234.04 −4642.01 −239483 −1040.43 −1136542 −1829152
(J mol −1 K −1 ) 29.85 28.82 18.54 24.79 0 132.51
C p
C × 10 −5 −1.674 0.502 −6.903 0 0
B × 10 3 4.184 3.263 42.284 12.38 35.14 Thermodynamic Data of Pure Species Involved in the Operation of an Aluminum-Air Power Source
29.96 27.28 10.669 20.67 120.8
A
S 0 (298 K) ) (J mol −1 205 131 69.9 28.325 96.86
G 0 (298 K) ) (J mol −1 0 0 −237000 0 −1136542 −1825500
Species O 2 H 2 H 2 O Al Al(OH) 3 Al 2 O 3 ·H 2 O TABLE 4.3