Page 78 - Corrosion Engineering Principles and Practice
P. 78
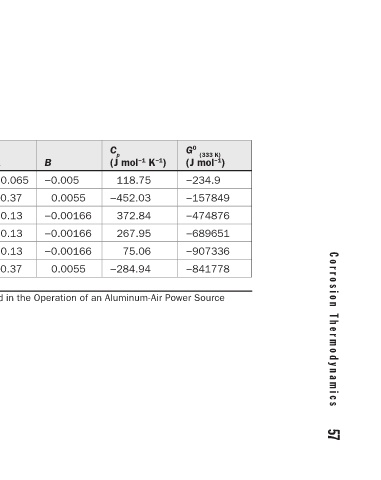
56 C h a p t e r 4 C o r r o s i o n T h e r m o d y n a m i c s 57
G 0 (333 K) ) (J mol −1 −234.9 −157849 −474876 −689651 −907336 −841778
(J mol −1 K −1 ) 118.75 −452.03 372.84 267.95 75.06 −284.94
C p
−0.005 0.0055 −0.00166 −0.00166 −0.00166 0.0055
B
0.065 −0.37 0.13 0.13 0.13 −0.37 Thermodynamic Data of Soluble Species Involved in the Operation of an Aluminum-Air Power Source
A
(298 K) ) (J mol −1 −20.9 20.968 −384.45 −184.06 184.43 117.31
Š 0
S 0 (298 K) ) (J mol −1 0 41.888 −321.75 −142.26 205.35 96.399
G 0 (298 K) ) (J mol −1 0 −157277 −485400 −694100 −900000 −838968
Species H + OH – Al 3+ Al(OH) 2+ + Al(OH) 2 – AlO 2 TABLE 4.4