Page 761 - Corrosion Engineering Principles and Practice
P. 761
712 A p p e n d i x A H i s t o r i c a l P e r s p e c t i v e 713
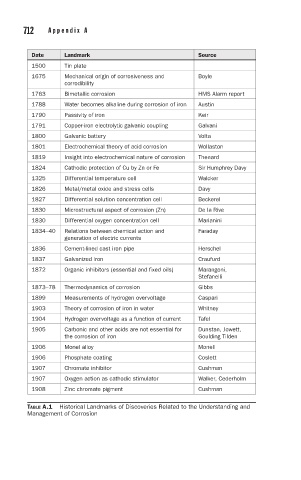
Date Landmark Source
1500 Tin plate
1675 Mechanical origin of corrosiveness and Boyle
corrodibility
1763 Bimetallic corrosion HMS Alarm report
1788 Water becomes alkaline during corrosion of iron Austin
1790 Passivity of iron Keir
1791 Copper-iron electrolytic galvanic coupling Galvani
1800 Galvanic battery Volta
1801 Electrochemical theory of acid corrosion Wollaston
1819 Insight into electrochemical nature of corrosion Thenard
1824 Cathodic protection of Cu by Zn or Fe Sir Humphrey Davy
1325 Differential temperature cell Walcker
1826 Metal/metal oxide and stress cells Davy
1827 Differential solution concentration cell Beckerel
1830 Microstructural aspect of corrosion (Zn) De la Rive
1830 Differential oxygen concentration cell Marianini
1834–40 Relations between chemical action and Faraday
generation of electric currents
1836 Cement-lined cast iron pipe Herschel
1837 Galvanized iron Craufurd
1872 Organic inhibitors (essential and fixed oils) Marangoni,
Stefanelli
1873–78 Thermodynamics of corrosion Gibbs
1899 Measurements of hydrogen overvoltage Caspari
1903 Theory of corrosion of iron in water Whitney
1904 Hydrogen overvoltage as a function of current Tafel
1905 Carbonic and other acids are not essential for Dunstan, Jowett,
the corrosion of iron Goulding Tilden
1906 Monel alloy Monell
1906 Phosphate coating Coslett
1907 Chromate inhibitor Cushman
1907 Oxygen action as cathodic stimulator Walker, Cederholm
1908 Zinc chromate pigment Cushman
TABLE A.1 Historical Landmarks of Discoveries Related to the Understanding and
Management of Corrosion