Page 765 - Corrosion Engineering Principles and Practice
P. 765
A p p e n d i x B
716
Helium
Argon
Neon
He
Ne
20.2
Ar
4.0
18
10
18
2
–1
–1
Fluorine
Chlorine
Cl
19.0
F
17
17
9
–2
+4
+6
–2
Sulphur
Oxygen
16.0
O
S
16
16
8
Phosphorus
–3
+5
Nitrogen
–3
14.0
P
N
15
15
7
+4
+4
+2
+2
Carbon
Silicon
12.0
Si
C
14
14
6
Aluminum
+3
+3
Boron
Al
10.8
B
13
13
5
12
11
10
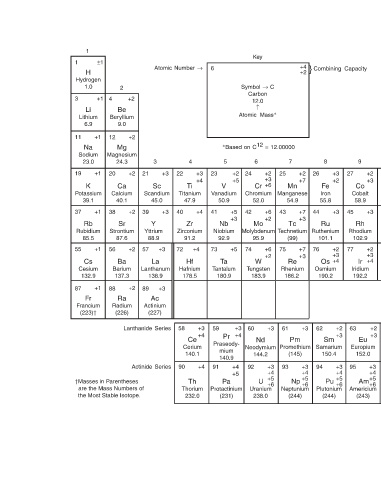
+2 40.0 35.5 32.1 31.0 28.1 27.0 36 –1 35 –2 34 –3 33 +4 32 +3 31 +2 30 +1 29 +2 28 +4 +5 +2 +2 +3 +3 +6 Kr Br Se As Ge Ga Zn Cu Ni Co Krypton Bromine Selenium Arsenic Germanium Gallium Zinc Copper Nickel Cobalt 83.8 79.
} Combining Capacity 8 27 +3 +2 Fe Iron 55.8 45 +3 Ru Ruthenium 101.1 77 +2 +3 +4 Os Osmium 190.2 63 +2 +3 Sm Samarium 150.4 95 +3 +4 Pu +5 +6 Plutonium (244)
9
+4 +2 7 26 +2 25 +7 Mn Manganese 54.9 44 +7 43 +3 Tc (99) 76 +7 75 +3 Re Rhenium 186.2 62 +3 61 Pm (145) 94 +3 93 +4 Np +5 +6 Neptunium (244)
Symbol → C Carbon ↑ ∗ Based on C 12 = 12.00000 +2 +3 +6 Cr Chromium 52.0 +6 +2 Mo Molybdenum Technetium 95.9 +6 +2 W Tungsten 183.9 +3 Nd Neodymium Promethium 144.2 +3 +4 +5 U +6 Uranium 238.0
Key 12.0 Atomic Mass ∗ 6 24 42 74 60 +3 +4 92
5
23 +2 +5 V Vanadium 50.9 +5 41 +3 Nb Niobium 92.9 +5 73 Ta Tantalum 180.9 59 Pr Praseody- mium 140.9 +4 91 +5 Pa Protactinium (231)
6 +3 +4 +4 +4 +3 +4 +4
Atomic Number → 4 22 +3 Ti Titanium 47.9 40 +3 Zr Zirconium 91.2 72 +3 Hf Hafnium 178.5 +3 58 Ce Cerium 140.1 90 Th Thorium 232.0
3
21 Sc Scandium 45.0 39 Y Yttrium 88.9 57 La Lanthanum 138.9 89 Ac Actinium (227) Lanthanide Series Actinide Series
+2 +2 +2 +2 +2 +2
Be Beryllium Mg Ca Sr Ba Barium Ra
2 9.0 Magnesium 24.3 Calcium 40.1 Strontium 87.6 137.3 Radium (226)
4 12 20 38 56 88 †Masses in Parentheses are the Mass Numbers of the Most Stable Isotope.
±1 +1 +1 +1 +1 +1 +1
1 H Hydrogen 1.0 Li Lithium 6.9 Na Sodium 23.0 K Potassium 39.1 Rb Rubidium 85.5 Cs Cesium 132.9 Fr Francium (223)†
1 3 11 19 37 55 87