Page 756 - Corrosion Engineering Principles and Practice
P. 756
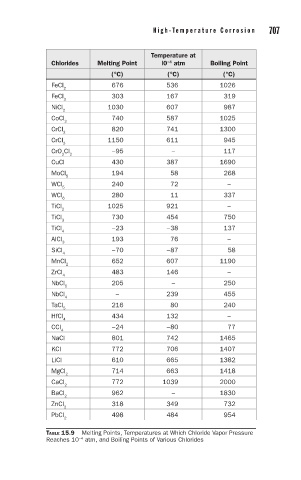
706 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 707
Temperature at
Chlorides Melting Point l0 atm Boiling Point
−4
(°C) (°C) (°C)
FeCl 676 536 1026
2
FeCl 303 167 319
3
NiCl 1030 607 987
2
CoCl 740 587 1025
2
CrCl 820 741 1300
2
CrCl 1150 611 945
3
CrO Cl −95 – 117
2 2
CuCl 430 387 1690
MoCl 194 58 268
5
WCl 240 72 –
5
WCl 280 11 337
6
TiCl 1025 921 –
2
TiCl 730 454 750
3
TiCl −23 −38 137
4
AlCl 193 76 –
3
SiCl −70 −87 58
4
MnCl 652 607 1190
2
ZrCl 483 146 –
4
NbCl 205 – 250
5
NbCl – 239 455
4
TaCl 216 80 240
5
HfCl 434 132 –
4
CCl −24 −80 77
4
NaCl 801 742 1465
KCl 772 706 1407
LiCl 610 665 1382
MgCl 714 663 1418
2
CaCl 772 1039 2000
2
BaCl 962 – 1830
2
ZnCl 318 349 732
2
PbCl 498 484 954
2
TABLE 15.9 Melting Points, Temperatures at Which Chloride Vapor Pressure
Reaches 10 atm, and Boiling Points of Various Chlorides
−4