Page 752 - Corrosion Engineering Principles and Practice
P. 752
702 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 703
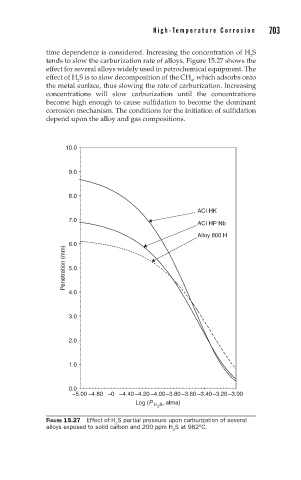
time dependence is considered. Increasing the concentration of H S
2
tends to slow the carburization rate of alloys. Figure 15.27 shows the
effect for several alloys widely used in petrochemical equipment. The
effect of H S is to slow decomposition of the CH , which adsorbs onto
2
4
the metal surface, thus slowing the rate of carburization. Increasing
concentrations will slow carburization until the concentrations
become high enough to cause sulfidation to become the dominant
corrosion mechanism. The conditions for the initiation of sulfidation
depend upon the alloy and gas compositions.
10.0
9.0
8.0
ACI HK
7.0
ACI HP Nb
Alloy 800 H
6.0
Penetration (mm) 5.0
4.0
3.0
2.0
1.0
0.0
–5.00 –4.80 –0 –4.40 –4.20 –4.00–3.80 –3.60 –3.40–3.20 –3.00
Log (P H 2 S , atma)
FIGURE 15.27 Effect of H S partial pressure upon carburization of several
2
alloys exposed to solid carbon and 200 ppm H S at 982°C.
2