Page 748 - Corrosion Engineering Principles and Practice
P. 748
698 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 699
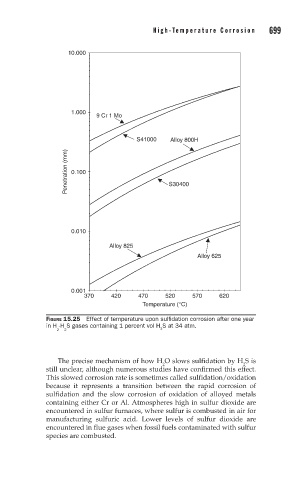
10.000
1.000
9 Cr 1 Mo
S41000 Alloy 800H
Penetration (mm) 0.100 S30400
0.010
Alloy 825
Alloy 625
0.001
370 420 470 520 570 620
Temperature (°C)
FIGURE 15.25 Effect of temperature upon sulfidation corrosion after one year
in H -H S gases containing 1 percent vol H S at 34 atm.
2 2 2
The precise mechanism of how H O slows sulfidation by H S is
2
2
still unclear, although numerous studies have confirmed this effect.
This slowed corrosion rate is sometimes called sulfidation/oxidation
because it represents a transition between the rapid corrosion of
sulfidation and the slow corrosion of oxidation of alloyed metals
containing either Cr or Al. Atmospheres high in sulfur dioxide are
encountered in sulfur furnaces, where sulfur is combusted in air for
manufacturing sulfuric acid. Lower levels of sulfur dioxide are
encountered in flue gases when fossil fuels contaminated with sulfur
species are combusted.